APPLICATION OF INNOVATIVE SORBENTS IN THE REMOVAL
OF CESIUM FROM RADIOACTIVE ALKALINE
DEFENSE WASTES
Douglas W. Hendrickson, P.E., Rabindra K. Biyani, P.E., and James B.
Duncan, Ph.D
SGN Eurisys Services Corp.
Richland, Washington
ABSTRACT
During 1996 and 1997 the U.S. Department of Energy (EM-50), through its Tank Focus Area, sponsored bench-scale flow testing of cesium sorbents with actual Hanford site tank wastes. Sorbents tested include: Resorcinol-Formaldehyde developed by Westinghouse Savannah River Co., and produced by Boulder Scientific; IONSIV™ IE-911 Crystalline Silicotitanate developed by Texas A&M University and Sandia Laboratory and produced in engineered form by UOP; and SuperLig™ 644 developed and produced by IBC Advanced Technologies, Inc.. Conduct of the flow tests required disposable equipment design and fabrication, acquisition and preparation of large volumes of tank wastes, and gamma energy effluent monitoring for breakthrough.
Alkaline tank wastes treated during the span of these tests include Hanford wastes identified as Double-Shell Slurry Feed (DSSF), Complexant Concentrate (CC), and saltcake from a total of five waste tanks. All waste materials were prepared by dissolution with water or sodium hydroxide to target concentrations of 5 M sodium. As prepared, the average DSSF feed concentration of 137Cs was 7.9 x 106 Bq/mL (~5.6 x 10-5 M total cesium)a. The average CC feed concentration of 137Cs was 5.6 x 106 Bq/mL (~4.3 x 10-5 M total cesium). The saltcake dissolutions yielded 137Cs concentrations of approximately 2.3 x 106 Bq/mL, 1.8 x 106 Bq/mL, and 5.7 x 106 Bq/mL (241-U-108, 241-U-109, and 241-A-101, respectively) and overall cesium concentrations estimated at 5 x 10-5 M.
Through the successful completion of the tests, significant lessons were acquired in materials handling, data collection, and sample handling. Of particular interest, on-line gamma energy detection enabled rapid response capabilities in interpreting shielding deficiencies, any necessary sample acquisition frequency changes, and the test completion as measured by breakthrough.
During 1996, the DSSF was contacted with Resorcinol-Formaldehyde regenerable resin and
IONSIV™ IE-911 with 50 percent breakthrough (l 50)
yields of 14 and 696 column volumes (CV), respectively. During 1997, IONSIV™ IE-911
contact of CC and saltcake wastes
yielded l 50 at 1,044 CV for CC wastes, and
approximately 570 and 960 CV from two saltcake wastes. Similarly, 1997 contact of
SuperLig™ 644 yielded l 50 at
approximately 189 CV for saltcake waste and 120 CV for CC waste. Complete elution of
the SuperLig™ 644 required 11.1 CV of 0.5 M HNO3 at 4 CV/hr for CC waste
and approximately 6 CV for the saltcake.
Of the materials tested, only one was determined to be unacceptable for the desired application. Both the nonregenerable crystalline silicotitanate and regenerable SuperLig™ 644 demonstrated high loading and stable performance with the highly alkaline brines over the period of test. Both of these materials have a high cesium collection efficiency and capacity lending themselves well to economical application in separations for the wastes types.
INTRODUCTION
During 1996 and 1997 the U.S. Department of Energy (EM-50), through its Tank Focus Area, sponsored bench-scale flow testing of cesium sorbents with actual Hanford site tank wastes. Sorbents tested include: Resorcinol-Formaldehyde (R-F) developed by Westinghouse Savannah River Co., and produced by Boulder Scientific; IONSIV™ IE-911 Crystalline Silicotitanate developed by Texas A&M University and Sandia Laboratory and produced in engineered form by UOP; and SuperLig™ 644 developed and produced by IBC Advanced Technologies, Inc.. Conduct of the flow tests required activities, such as equipment design for direct process scale-up, that could be directly loaded through airlocks to available hot cells, acquisition and preparation of large volumes of tank wastes, and gamma energy effluent monitoring for breakthrough.
Alkaline tank wastes treated during the span of these tests include Hanford wastes identified as Double-Shell Slurry Feed (DSSF), Complexant Concentrate (CC), and saltcake from a total of five waste tanks.
The work was designed to address interests, including U.S. Department of Energy (DOE) site operations in sorbent material specification, waste characterization, process design and instrumentation, and waste management. Although the waste materials tested differ significantly from most commercially contaminated liquid streams, the process design, instrumentation, and sorbent material specification may be of value to commercial entities. Additional applications may be in brine component separation.
DSSF material was acquired, at a volume of approximately five liters, from tank
241-AW-101 and diluted with water from 10 M sodium to a target of 5 M
sodium. As prepared, the average feed concentration of 137Cs was
7.9 x 106 Bq/mL (~5.6 x 10-5 M total
cesium). CC waste material was acquired, at a volume of approximately five liters,
from tank 241-AN-107 and diluted with aqueous sodium hydroxide to 5 M sodium
from 8.5 M sodium. As prepared, the average CC feed concentration of 137Cs
was 5.6 x 106 Bq/mL (~4.3 x 10-5 M
total cesium). Saltcakes were acquired in individual jars from previous core drilling
efforts of tanks. The saltcakes were removed from the jars and dissolved in aqueous sodium
hydroxide to target concentrations of 5 M sodium from approximately 20 M.
As prepared, the saltcake dissolutions yielded 137Cs concentrations of
approximately 2.3 x 106 Bq/mL, 1.8 x 106
Bq/mL, and 5.7 x 106 Bq/mL (241-U-108, 241-U-109, and 241-A-101,
respectively) and overall cesium concentrations estimated at
5 x 10-5 M.
Through the successful completion of the tests, significant lessons were acquired in materials handling, data collection, and sample handling. Of particular interest, on-line gamma energy detection enabled rapid response capabilities in interpreting shielding deficiencies, any necessary sample acquisition frequency changes, and the test completion as measured by breakthrough.
SORBENTS TESTED
Several cesium removal sorbents have been developed by private industry and the DOE's Office of Science and Technology (OST) [EM-50] for the removal of cesium from the radioactive tank wastes located at various DOE facilities. In mid-1996, ion exchange column tests were conducted for evaluating cesium removal from Hanford's DSSF waste in tank 241-AW-101 (Ref. 1). There have also been a number of batch tests using Hanford tank wastes and several other column tests using simulated Hanford tank wastes or actual wastes from other DOE sites.
Resorcinol Formaldehyde
The R-F resin is an organic condensation resin anticipated to provide significant selectivity for cesium as a result of the cross-linking and swelling characteristics of the resin. Based upon prior Pacific Northwest National Laboratory (PNNL) experience, 50 percent breakthrough of this material was anticipated to be in the range of 40 to 50 CV.
In this test, the R-F resin was from a batch identified as SRS-B material. The resin was manufactured by Boulder Scientific Company (Boulder, CO) in 1988; no batch number was provided by the TFA with this sorbent. The material, as received, was in its potassium form with a particle size range of -60+80 mesh.
In batchwise preparation of the material, the resin was eluted with 10 CV of 0.5 M HNO3, rinsed with 10 CV of deionized water, and conditioned with 10 CV of 2.5 M NaOH.
The conditioned material was slurried into the test columns by calibrated micropipette and allowed to settle to predetermined bed heights of ten cm, thereby defining the column volume as the bed volume of conditioned resin. Bed retainers were emplaced limiting potential bed expansion to approximately 12 cm. The gap between bed and bed retainer remained fluid filled.
Crystalline Silicotitanate
The CST material was provided by UOP, from their Chickasaw, AL, molecular sieve plant. The material is produced by UOP as IONSIV™ IE-911b, and is now commercially available. The material tested in 1996 was from Lot No. 999096810001, and received by WHC on May 15, 1996. The material tested in 1997 was from Lot No. 999096810002, and received by SESC in November 1996.
Crystalline silicotitanates (CST) are a new class of inorganic ion exchangers invented and developed by Sandia National Laboratories (SNL) and Texas A & M University in 1992. It was determined that CSTs have a high affinity for Cs and Sr ion exchange in highly alkaline solutions. Based upon this finding, an extensive program funded by Laboratory Directed Research and Development at SNL, the Efficient Separations Program, and the Tank Waste Remediation System was established. As the development proceeded, a Cooperative Research and Development Agreement was executed with UOP (DesPlaines, IL) to scale up CST production to commercial quantities and to develop an engineered form for use in ion exchange columns. Extensive input was obtained from WHC, PNNL, and DOE on the required properties of the engineered form for use at Hanford. Based upon this guidance, a 30/60 mesh ion exchanger called IONSIV™ IE-911 was developed by UOP, tested at Sandia, and made available to WHC and other DOE facilities.
The IONSIV™ IE-911 was prepared, in batch mode, by wetting the ion exchanger and removing the fines. A pretreatment step to adjust the pH of the ion exchanger to be in equilibrium with the waste solution was completed in batch mode with sodium hydroxide. The conditioned material was slurried into the test columns by calibrated micropipette and allowed to settle to predetermined bed heights of ten cm in 1996 work and seven or ten cm in 1997 (lead versus guard columns). Additional conditioning with sodium hydroxide preceded waste contact in flow tests.
Superlig™ 644
The SuperLig™ 644c material was received from IBC Technologies, Inc. of American Fork, Utah, in March 1997. No batch or production identifiers were provided with the material.
The exchange material was prepared, in batch mode, by wetting the ion exchanger and removing the fines and floaters. A pretreatment step to adjust the pH of the ion exchanger to be in equilibrium with the waste solution was completed in flow with sodium hydroxide after the material was slurried into the test columns by calibrated micropipette and allowed to settle to predetermined bed heights of approximately 10 cm for each column. These bed depths define the CV for these tests as 7.85 mL. The volume of exchange material in each guard column provided an additional bed volume of treatment. Following conditioning, the system was water washed to near neutrality to minimize any degradation potential pending hot cell entry.
EXPERIMENTAL SYSTEMS AND APPARATI
Hot cell Facilities
Hot cell facilities for test conduct were provided at the 222-S Analytical Laboratory in the 200W Area of the Hanford Site. Test conduct was optimal here as routine procedures for tank waste sampling required that materials be processed through this laboratory and wastes from analysis and test conduct may be directly flushed to the tank system source of the test material. During 1996 these facilities were operated by Westinghouse Hanford Company; contract transition of this DOE site led to laboratory operation by Numatec Hanford Corporation and Rust Federal Services Hanford, Inc. for predominant material handling and analytical support.
The conduct of tests within hot cells allowed far more extensive testing than had previously been conducted, but did require that significant design and procedural details be explicitly met. Included in such considerations are the baseline equipment design for test apparati allowing intact and fully assembled test equipment to be loaded into the hot cell through the sample airlock.
Gamma Detection System
A cadmium zinc telluride gamma detection system (Ref. 2) was employed in these tests, recognizing that ion exchange performance measurement might be significantly enhanced by essentially constant measurement rather than the more typical experimental profile containing periodic samples and wet chemistry analyses. In concert with periodic samples, the gamma detection system employed allowed determination of early breakthrough and performance measurement in quantitative terms.
The gamma detector was prepared for continuous monitoring of the effluent during each phase of the test. The detector acquired gamma energy data from the test apparatus and flows and recorded the data on an IBMd compatible computer using the GammaVisione program. In addition to breakthrough monitoring, the detector was applicable for purge and rinse observation following the R-F test conduct and elute observation of SuperLig™ 644.
Column Flow Assemblies
Each test conduct required the construction of an apparatus substrate containing glass test columns, valves, and a peristaltic pump. All loaded columns were designed to minimize wall effects to allow direct process scaleup. Peristaltic pumps were applied to mitigate material difficulties associated with the variable and highly corrosive characteristics of the waste solutions and eluates (nitric acid). The peristaltic motors and feed line diameters were chosen to maintain target flows in the motor midrange. Test equipment was designed, built, and installed as disposable equipment. The test apparati consisted of a Plexiglasf basin and upright back piece to retain the various valves, and columns and the peristaltic pump. The valves were of aluminum body construction with Teflong cores and barbs. Valves and their handles were selected for frequency of use and hot cell manipulator operation. Flow lines were composed of Tygonh tubing to facilitate disposal. Following test conduct, apparati were disassembled and melted into waste cans to minimize waste volume.
Resorcinol Formaldehyde/Crystalline Silicotitanate: The 1996 work with DSSF waste solution was conducted in a test assembly with two parallel sets of lead and guard columns containing the same exchange material. All columns had an ID of one cm. In this assembly, R-F resin was contained in the "A" subassembly while CST was contained in the "B" subassembly. Each lead column had a wetted bed height of 10 cm.
Crystalline Silicotitanate with Dual Guard: The 1997 CC waste and saltcake waste treatments with CST were conducted in test assemblies constructed with two parallel lead columns sharing oversize guard columns. The lead columns had IDs of 0.7 cm, guard columns were of one cm ID, and all columns were filled to a height of 10 diameters.
SuperLig™ 644: The 1997 CC waste and saltcake waste treatments were conducted in a test assembly with two parallel sets of lead and guard columns containing the same SuperLig™ 644 exchange material. All columns had an ID of one cm and were filled to a wetted bed depth of 10 cm. The elution line provided the primary difference between this apparatus and that used in 1996. The elution line was crafted to provide sufficient decay time to allow experimenters to observe the eluted sorbate with on-line gamma spectrometry.
TREATMENT LIQUORS
Double-Shell Slurry Feed Liquor
DSSF liquor is evaporatively concentrated brine from Hanford defense waste operations. These wastes are of miscellaneous origins and are concentrated to, in this case, 10 M sodium. This waste has modest concentrations of organic constituents and minor carbonate concentrations. In this test work, the material was acquired from tank 241-AW-101 in the 200E Area of the Hanford site. The material was diluted with water and resulted in a solution containing an average of 5.5 M Na, 0.5 M K, 0.57 M Al, 2.7 M OH-, 0.11 M CO32-, 1.13 M NO2-, and 1.4 M NO3-.
Complexant Concentrate Liquor
CC wastes are brines containing greater than 10 wt. percent organic material and contain significant levels of complexant materials used for material separations in Hanford operations. The CC waste used in these tests was acquired from tank 241-AN-107 in the 200E Area of the Hanford site. As acquired, the material had concentrations of 8.5 M Na and 1.21 x 105 Bq/mL 137Cs. Significant concentrations of carbonate ion were anticipated to interfere in cesium sorbtion. As prepared, the feed material was 5.19 M Na and 0.126 M OH-. Other anions had average molar concentrations of 0.095 for fluoride, 0.026 for chloride, 0.655 for nitrite, 1.95 for nitrate, 0.008 for phosphate, and 0.05 for sulfate (Ref. 3). Analysis and discussion described in (Ref. 3) established the prepared CC feed solution concentration as 166.6 µCi/mL 137Cs. The isotopic analysis of CC waste yielded 137Cs as 32.94 mole percent.
Dissolved Salt Cake Liquors
Saltcake wastes are the evaporatively concentrated crystallized salt wastes resulting from Hanford defense waste operations. These materials are predominantly sodium nitrate, sodium nitrite, and other salts. These materials must be dissolved for cesium ion exchange tests. In this work saltcakes were derived from three tanks on the Hanford site for test conduct and dissolved with aqueous sodium hydroxide to acquire 5 M Na, 0.5 M OH- solutions.
EXPERIMENTAL RESULTS
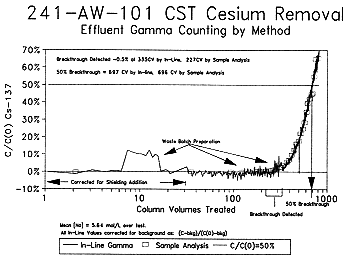
R-F and CST Contact with DSSF
Conclusions of the bench-scale contact of R-F and CST IONSIV™ IE-911 with actual tank waste under conditions anticipated to be seen in full scale processes are:

Figure 1. CST Test On-line Gamma Detection Vs. Wet Chemistry.
CST and Superlig™ 644 contact with CC
CC waste from Hanford Double-Shell Tank 241-AN-107 was successfully sampled from the tank, prepared, treated with CST, and sampled for cesium removal in a bench-scale radioactive flow test (Ref. 3). On-line gamma energy analyses of treatment effluents yielded high selectivities and load of cesium upon IE-911™ crystalline silicotitanate exchange material. Beta radiation detected upon sample load out indicated that strontium was not significantly removed from the waste. (Fig. 2)
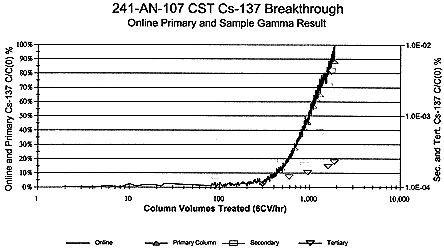
Figure 2. 241-AN-107 137Cs Breakthrough Over IE-911 CST (6CV/hr).
CC wastes from Hanford tank 241-AN-107 were successfully treated with SuperLig™ 644 and sampled for cesium removal in bench-scale radioactive flow tests (Ref 4). On-line gamma energy analyses of treatment effluents yielded modest selectivities and load of cesium upon IBC Technologies, Inc., SuperLig™ 644 sequestrant. (Fig. 3)
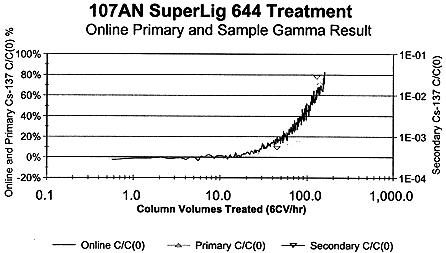
Figure 3. 241-AN-107 137Cs Breakthrough Over SuperLigTM 644 (6CV/hr)
CST and Superlig™ 644 Contacts with Saltcake Dissolutions
Saltcake wastes from Hanford Single-Shell Tanks 241-U-108, 241-U-109, and 241-A-101 were successfully transferred, prepared, treated with CST, and sampled for cesium removal in bench-scale radioactive flow tests (Ref. 5). On-line gamma energy analyses of treatment effluents yielded high selectivities and modest load of cesium upon IE-911 crystalline silicotitanate exchange material.
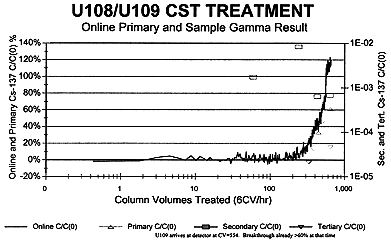
Figure 4. 241-U-108/241-U-109 Cesium Removal with CST.
Saltcake wastes from Hanford tank 241-U-109 were successfully treated with SuperLig™ 644 and sampled for cesium removal in bench-scale radioactive flow tests (Ref. 4). On-line gamma energy analyses of treatment effluents yielded modest selectivities and load of cesium upon IBC Technologies, Inc., SuperLig™ 644 sequestrant.
Elution of R-F and Superlig™ 644
SUMMARY
During 1996, the DSSF was contacted with R-F regenerable resin and IONSIV™ IE-911 with 50 percent breakthrough yields of 14 and 696 CV, respectively. In 1997 work, IONSIV™ IE-911 contact of CC and saltcake wastes yielded 50 percent breakthrough at 1,044 CV for CC wastes, and 570 and 960 CV for the saltcake wastes. Similarly, 1997 contact of SuperLig™ 644 yielded 50 percent breakthrough at approximately 189 CV for saltcake waste and 120 CV for CC waste. Complete elution of the R-F required 10 CV of 0.5 M HNO3 at 2 CV/hr. Complete elution of the SuperLig™ 644 required 11.1 CV of 0.5 M HNO3 at 4 CV/hr for CC waste and approximately 6 CV for the saltcake.
Of the materials tested, only one was determined to be unacceptable for the desired application. Both the nonregenerable crystalline silicotitanate and regenerable SuperLig™ 644 demonstrated high loading and stable performance with the highly alkaline brines over the periods of test. Both of these materials have a high cesium collection efficiency and capacity lending themselves to economical application in separations for the waste types. Applications of these materials, as functions of the reported tests, to particular processes may be available.
Notes
REFERENCES