MORPHOLOGY AND MASS REDUCTION OF ORGANIC ION
EXCHANGE RESINS IN PYROLYSIS/OXIDATIVE
PYROLYSIS PROCESS
Kwansik Choi*, Kyung-Hwa Yang, Ung-Kyung Chun , Jong-kil Park
and Myung-Jae Song
Korea Electric Power Research Institute
103-16, Munji-Dong, Yusung-gu, Taejon, 305-380, Korea
*Tel. (82-42) 865-5727, Fax (82-42) 865-5704, email: kschoi@kepri.re.kr
ABSTRACT
Pyrolysis may be an important pretreatment step before vitrification in a cold crucible melter (CCM). During vitrification of organic ion exchange resin the carbon or other remaining residues may harm the performance of the cold crucible melter or the eventual stability of the final glass product. Hence, it is important to reduce or prevent such harmful waste from entry into the cold crucible melter. Pretreatment with pyrolysis will generally provide volume reduction resulting in less amount of solid waste that needs to be handled by the CCM. Hence, KEPRI has undertaken studies on the pyrolysis and oxidative pyrolysis of organic ion exchange resin.
Pyrolysis and oxidative pyrolysis were examined with the thermogravimetric analysis (TGA) and a tube furnace. TGA results for pyrolysis with the flow of nitrogen indicate that even after pyrolyzing from room temperature to about 900°C, a significant mass fraction of the original cationic resin remains, approximately 46%. The anionic resin when pyrolytically heated in a flow of nitrogen only, from room temperature to about 900°C, produced a final residue mass fraction of about 8 percent. Oxidative pyrolysis at a ratio of air to nitrogen, 1:2, reduced the cationic resin to 5.3% of the original mass when heated at 5°C/min. Oxidative pyrolysis of the anion left virtually no residue. Tube furnace experiments were performed to obtain larger samples of residue. The morphology of the residue, analyzed with an SEM, reveals different morphologies arising between the anionic and cationic resin from the different chemical mechanisms involved in the breakdown processes.
INTRODUCTION
Pyrolysis may be an important pretreatment step before vitrification in a cold crucible melter (CCM). Waste characteristics play an important role for the vitrificaton considersations. During vitrification of organic resin the carbon or other remaining residues may harm the performance of the cold crucible melter or the eventual stability of the final glass product. Hence, it is important to reduce or prevent such harmful waste from entry into the cold crucible melter. Pretreatment with pyrolysis will generally provide volume reduction resulting in less amount of solid waste that needs to be handled by the CCM; in addition, the pyrolytic processes may breakdown much of the complex organics causing release through volatilization resulting in less carbon and other harmful substances. Oxidative pyrolysis may be utilized to further reduce the quantity of residue.
Past Work on the Pyrolysis of Organic Resin
Understanding the waste characteristics is essential for any pretreatment. The resin used in the experimentations at KEPRI are the resin used in Korean nuclear power plants: Amberlite IRN77 (cationic) and Amberlite IRN78 (anionic). Neely of Rohm and Haas, the supplier of IRN77 and IRN78, has characterized similar polymer carbons derived from porous sulfonated polystyrene (1). Others have examined anionic ion exchange resins and noted that after pyrolysis, the residue from these resins is generally very small (e.g. less than 5%).
The thermal decomposition of polystyrene has been shown to proceed by a combination of depolymerization (unzipping) and chain scission reactions (2). The first observable weight loss occurs at 260°C in air and 350°C in nitrogen with complete volatilization by 440°C. Crosslinked polysyrenes convert readily to carbonaceous residues between 300 and 500°C indicating condensation processes competing with chain scission (3). The yield of carbonaceous solid increases with 50% divinylbenzene giving a residue equivalent to 6% of the original skeleton and 100% trivinylbenzene giving about a 55% weight yield (3). The weight yield of 100% DVB is increased from 8% to about 80% by carbonization at high pressure (4).
Neely found when examining the highly acidic cationic resins with a sulfonic group that microporosity is created during heat treatment and as the temperature rises the volume of micropores increases and the effective size of the micropores decreases(1). The micropore volume increases linearly as the skeletal volume decreases with half the volume lost be the skeleton creating new micropores and the other half appearing as shrinkage of the bead.
There has been experience on resin pyrolysis by various researchers.
Peterson and Kemmler
Peterson and Kemmler carried out the pyrolysis of powder resins in a pilot system with a capacity of about 30 kg/hr (5). The resin was first pre-treated by drying such that 50% of its weight was reduced. The resins were heated around 300-350°C and the weight reduction was about 90% based on 65% water content of the starting material. The residues or ash was found to mainly consist of carbon. Due to the low temperature of the pyrolysis process, the radioactive components remained in the residue.
Matsuda et. Al
They concluded that pyrolysis of spent ion exchange resin is one of the most effective methods for reducing radioactive waste volume and for making the final waste form more stable (6). Fundamental experiments were performed to clarify the pyrolysis characteristics of anion and cation exchange resins. Residual elemental analyses and off-gas analyses showed that the decomposition ratio of cation resins was only 50 wt % at 600°C, while that of anion resins was 90 wt% at 400°C. Infrared spectroscopy for cation resins attributed its low decomposition ratio to formation of a highly heat-resistant polymer (sulfur bridged) during pyrolysis. Measurements of residual hygroscopicity and cement package strength indicated that the optimum pyrolysis temperatures for preventing resin swelling and package expansion were between 300 and 500°C.
EXPERIMENTAL SET-UP
In addition to various TGA runs, a tube furnace was utilized for the pyrolysis of larger quantities of resin. The tube furnace experimentation allows us to examine some of the basic characteristics of waste at a small scale to help better design and understand what would happen in large scale experiments. In addition, even though the tube experiment processes are relatively small-scale in comparison to real scale, they allow observations of processes on a larger scale than the TGA. In addition, the bench-scale experiments also allow modifications in the experimental set-up that may not be allowed with the TGA.
General Set-Up
Nitrogen is fed in from one end of the tube furnace and exhausted out the other end. The samples to be pyrolyzed or oxidatively pyrolyzed were situated midway in the tube.
Tube Furnace
The tube furnace is a quartz tube furnace that is electrically heated along its walls. It is surrounded by alumina insulation. The tube diameter is about 7 cm while the length of the tube is roughly 90 cm. Temperature control is allowed at three axial positions of the tube furnace: one midway along the length of the tube and the other two equidistant (12 cm) from the midway controller. Two thermocouple probes are located midway along the length of the furnace. One probe is centered on the axis while the other probe is placed into the resin material.
Wastes
Amberlite-77, divinylbenzene-SO3H, and Amberlite-78, divinylbenzene-N(CH3)3OH, are the cation and anion resins, respectively. The size for resin balls vary between 0.3 mm and 1.2 mm. The average is roughly 0.55 mm. The polymer is styrene and divinyl benzene polymerized together. For cation, the active group is Poly-SO3-H. For anion the active group is Poly-N(CH3)3-OH. The density for cation is nominally 1.2 g/ ml. For anion, the density is 1.06 g/ml. The water content is nominally 58 to 60% for IRN78(anion) and for IRN77(cation), the water content is nominally 51 to 53%. The water in the bead is held in an equilibrium within the hydration zone of the charged exchange sites.
OPERATIONAL CONDITIONS.
Batches of resin were dried in a muffler furnace for 24 hr. at 100°C. The water content of about 51% correlates very well with that of prior known data. The resin was weighed using a mass balance.
The sample of resin, around 1.5 or 3 g, was placed at the center of the quartz tube in a boat shaped ceramic crucible. Nitrogen was continually fed into the furnace usually at 2 l/min. The temperatures were changed by varying the oven control conditions after the oxygen detection limits were near 0. Temperatures were recorded for the oven wall temperatures, centerline, and resin.
After each experiment, the quartz tube was cleaned with a solution of acetone. The acetone was generally effective in cleaning the tube. Heptane was also available for cleaning.
EXPERIMENTAL ANALYSIS
The following equipment were used for the experimental analysis: SEM, TGA, Mass Balance, and thermocouples.
RESULTS OF THE EXPERIMENTS
Many of the results/conclusions of the experiments with cations and anions of IRN-77 and IRN-78, respectively, can be substantiated by past research. Although the brand of organic ion exhange resin was not exactly the same as those used by past researchers, the functional groups and their characteristics are similar.
Several experiments were performed to understand the characteristics of cationic and anionic exchange resin. The data provide much information on the evolution of resin during various pyrolytic conditions.
TGA Results
A TGA provides analyses into the mass loss behavior of resins as a function of temperature for various ambient conditions. Heating of material can occur under pure pyrolytic conditions (e.g. pure nitrogen environment) or under oxidative conditions (e.g. with oxygen present). It is noteworthy to note that the TGA analyzes only a very small sample (e.g. approx. 10 mg) while typical pilot-scale waste pre-treatment units would pyrolyze relatively very large samples (e.g. in the several kilograms) at a time.
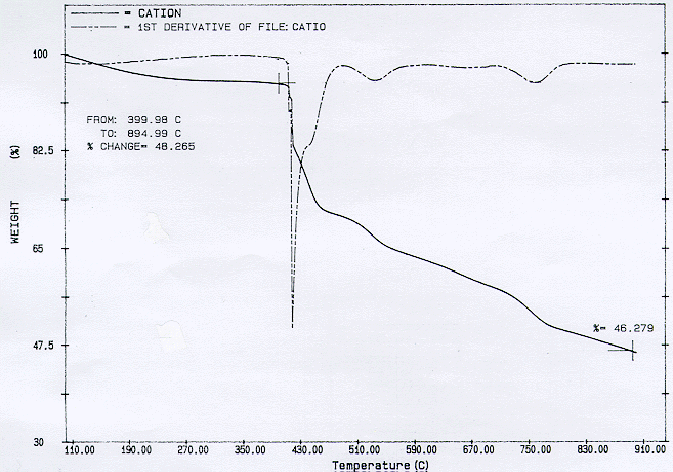
As shown in Fig. 1, TGA results for pyrolysis with the flow of nitrogen indicate that even after pyrolyzing from room temperature to about 900°C, still a significant mass fraction of the original cationic resin remains, approximately 46%. This is consistent with the results of Neely (1). This is most likely due to the formation of a highly heat resistant polymer (sulfur bridged) during pyrolysis. Matsuda et al. reported that a part of the functional sulfonic acid group would form sulfonyl bridges (-SO2-) between the base polymers at about 300°C (6). In the KEPRI TGA analysis, about 400°C was observed in contrast to 300°C. The difference is under investigation. This difference may partly be attributed to the use of slightly different.

Fig. 1. Pyrolysis of cationic exchange resin in a flow rate of 75 cc/min of nitrogen. The heating rate is 5oC/min.
A sample of the cation resin was oxidatively pyrolyzed. The flow rate through the pyrolysis chamber was comprised of 25 cc/min. of air with 50 cc/min of nitrogen. The fraction of original resin still left was approximately a significant 5.3% as shown in Fig. 2. There appear to be further oxidation at 400 °C for further significant release of volatiles at 400°C and then, also at around 800°C. Kinoshita et al., however, show in their TGA analysis of similar cations, almost no residue when heated to about 900°C (7). They state that the final decompostion entails combustion of the remaining base polymers. The absence of residue is likely due to the use of higher oxidant concentrations, 200 ml/min of air in contrast to 25 ml/min of air used in the KEPRI experiments.
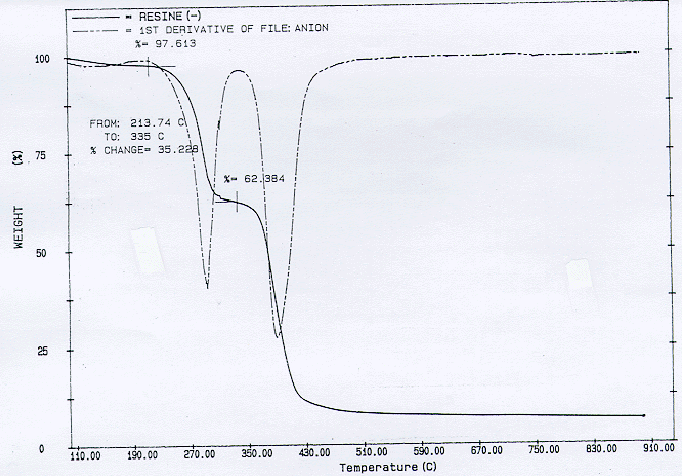
Fig. 2. Oxidative pyrolysis of cationic resin at 25 cc/min of air and 50 cc/min of nitrogen. The heating rate is 5 Figure 4. Oxidative pyrolysis of anionic resin at 25 cc/min of air and 50 cc/min of nitrogen. The heating rate is 5oC/min.
TGA analyses were perfomed on an 8.2 mg sample of anion. The specimen when pyrolytically heated in a flow of nitrogen only from room temperature to about 900°C produced a final residue mass fraction of about 8 percent. Fig. 3 shows a dramatic release of volatiles that start at about 200°C and 400°C. This agrees well with the data of Matsuda et. al (6). They implemented an anion resin which, too, was composed of a copolymer of styrene and divinylbenzene, and a functional group of quaternary ammonium. It is likely that the behavior of the volatilization of the sample can be explained from their observations as well. At 200°C, it is likely that methylamine gas was generated. They had noticed a decrease in the nitrogen content in the residue. This would indicate that the functional group would decompose at this temperature. Later, hydrocarbon gases would be generated since they had noticed that the carbon content in the residue decreased above 400°C, corresponding to the base polymer pyrolysis. For the oxidative pyrolysis trial where nitrogen was fed in at 50 cc/min and air at 25 cc/min, the resulting mass loss curve as shown in Fig. 4, shows a continual mass decrease until almost no residue is left. These trials are in stark contrast to the cation trials where significant amount of residue still remained.
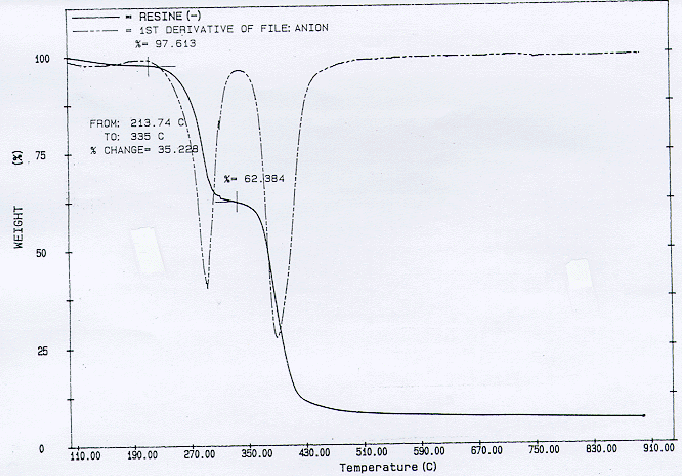
Fig. 3. Pyrolysis of anionic exchange resin in a flow rate of 75 cc/min of nitrogen. The heating rate is 5oC/min.
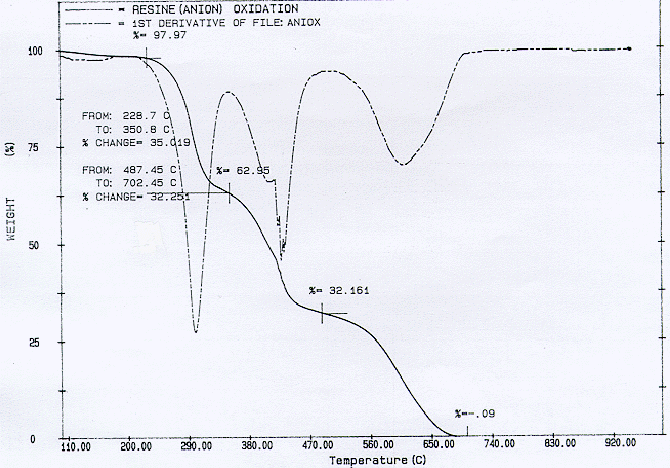
Fig. 4. Oxidative pyrolysis of anionic resin at 25 cc/min of air and 50 cc/min of nitrogen. The heating rate is 5 C/min.
Morphology
The understanding of morphology can be very helpful from the standpoint of understanding the pretreatment processes. From the morphology, some of the physical and chemical mechanisms are revealed. Tube furnace experiments were performed to provide samples for SEM analysis. From the SEM pictures alone, it was obvious that different decomposition mechanisms were at play. Fragmentation was observed in one while fracturing and the release of a porous medium from the fracture location was observed in the other. The anionic exchange resins were observed to dry, fragment, and disintegrate via volatilization. The cationic exchange resins were observed to fracture and from the fracture points, porous medium would spread outwards. Eventually the smooth shell of the cationic exchange resins would disappear and only the porous medium would be present. Pore sizes were first observed to increase with increase in temperature. The sponge-shaped morphology may be attributed to the sulfur bridging. With further increasing temperatures, the pore sizes were observed to shrink.
SEM pictures of the mixed resin (50 wt % anionic and 50 wt % cationic) showed that the final product was not a simple collection of morphologies of the anionic and cationic resin. There was a new morphology likely due to the interaction between the cationic and anionic resin.
CONCLUSION
Pyrolysis and oxidative pyrolysis were examined with TGA and a tube furnace. TGA results for pyrolysis with the flow of nitrogen indicate that even after pyrolyzing from room temperature to about 900°C, still a significant mass fraction of the original cationic resin remains, approximately 46%. The anionic resin when pyrolytically heated in a flow of nitrogen only, from room temperature to about 900°C, produced a final residue mass fraction of about 8 percent. Oxidation at a ratio of air to nitrogen, 1:2, reduced the cationic resin to 5.3% when heated at 5 C/min. Oxidation of anionic resin at the same ratio and same heating rate left almost no solid residue. Pyrolysis (e.g. nitrogen-only environment) in the tube furnace of larger samples provided samples for SEM analysis. SEM analysis of the cationic, anionic, and mixed resins show distinct morphologies for each. Many more tests are still required to examine the effects of mixed resin, excess air, heating rates, etc.
REFERENCES