DECONTAMINATION OF BERKELEY NUCLEAR POWER
STATION STEAM GENERATOR FINNED TUBING
TO FREE RELEASE
Thomas E. Morris
CORPEX Technologies Inc.
Research Triangle Park, NC
C. Michael Groom
Jordan Engineering UK Limited
Yate, Bristol, England
ABSTRACT
Jordan Engineering successfully applied the CORPEXÒ Nuclear Decontamination Process to decontaminate over 103 metric tons of finned tubing from a heat exchanger at the Berkeley Nuclear Power Station.
Earlier decontamination attempts with mineral acids, such as hydrochloric acid and other acid based solutions (e.g., fluoroboric acid), were unsuccessful in achieving the project goals and created safety concerns for the labor force. The CORPEXÒ Nuclear Decontamination Process employs near neutral pH, non-corrosive chemicals. Over 99 percent by weight of the metal was free released in a worker-safe environment.
INTRODUCTION
The Berkeley Nuclear Power Station was operated by Magnox Electric plc from 1962 through 1988. At the site, there were two High Temperature Gas-cooled Reactors (HTGR) with a nominal rating of 276 MWe each. These units were carbon dioxide cooled, graphite moderated, high temperature reactors. Each unit had 8 steam generators/heat exchangers. In 1988, the decision was made to place the HTGR units in shut-down mode and to decommission them. The units have had the interconnecting ducts and steam generators removed. The steam generators are laying on their side surrounding each reactor. The reactors are in a 30-year holding mode allowing for the decay of the short-lived radioactivity in the graphite moderator.
The scope of work contracted to Jordan Engineering was the complete disassembly and decontamination of one steam generator with the objective being the free release of all metal contained therein with minimal radioactive waste generation. Additional objectives included performing the decontamination under ALARP (As Low As Reasonably Possible, the UK equivalent of ALARA) conditions. Initial trials on the finned tubes, which made up the bulk of the contaminated tubing (>90% by weight relative to the plain tubing) demonstrated the difficulty in decontamination. Use of hot mineral acids, such as hydrochloric acid, and other acids, such as fluoroboric acid, were not effective in reducing the bulk radioactivity to less than 0.40 Bq/gram above background, the limit for free release of the carbon steel. Additionally, the dangers associated with hot acids to workers and equipment in a facility which was no longer completely functional were of concern from a safety standpoint.
DETAILS OF STEAM GENERATOR FINNED TUBING
At the initiation of the project, the total weight of finned tubing per steam generator was estimated to be 100 metric tons. After disassembly, the actual weight was determined to be close to 120 metric tons. Additional parts of the steam generator included approximately 10 metric tons of plain tubing and the shell of the steam generator. The total length of finned tubing is 55,000 feet with the following dimensions: Tube Inner Diameter: 0.845 inches, Tube Outer Diameter: 1.200 inches, Outer Diameter of Fins: 1.805 inches, Thickness of the Fins: 0.060 inches, and the gap between the Fins: 0.085 inches. The finned tube material is ERW Mild Steel BS1654.
All of the finned tubing was heavily scaled, both on the outside where the radioactive contamination was present and on the interior of the tubing. In addition to the hematite (red rust) on the outside of the scale, the tubing was coated with a black, hard, adherent carbonaceous deposit. The isotopes present were Co60 and Fe55. The field radiation survey meters were calibrated to measure the Co60 gamma levels on the steel. A safety factor of four was used to account for the Fe55, a beta emitter. Analytical work performed by the University of Bristol demonstrated very low levels of Fe55 after decontamination with the CORPEXÒ Process, therefore the safety factor was reduced to two, permitting field survey to determine free release levels of the steel. After the steel was chemically decontaminated, a three hour, high temperature (500° C) treatment of the metal was required to drive off any tritium contamination that may have migrated into the steel.
DESCRIPTION OF DECONTAMINATION CHEMICALS
CORPEXÒ -934 is a blend of surfactants, dispersants, detergents and anti-redeposition agents designed for removal of organic contamination from surfaces. CORPEXÒ -934 was used as the first step in the Process to remove and soften the carbonaceous coating so that the following steps could dissolve the isotopes of concern.
CORPEXÒ -921 is designed to dissolve the compounds of radioactive isotopes and the corrosion product matrices which bind them to surfaces. This neutral pH solution contains a patented chelation chemical which does not attack base materials, but rapidly dissolves corrosion products and binds radioactive isotopes, cleaning surfaces and preventing redeposition. This chemical dissolved the bulk of the corrosion products containing the cobalt and iron isotopes.
SMEARAWAY7 is a blend of non-ionic surfactants and has been used by the commercial and government nuclear industry for many years to remove radioactive, loose surface and particulate (smearable) contamination and prevent re-deposition. This chemical was used as a post-treatment to remove any lightly adhering residue due to dragout from the surface of the tubes.
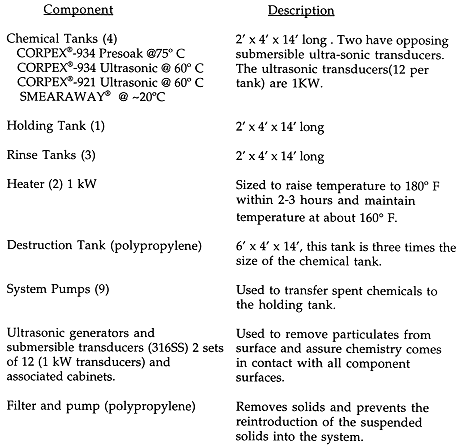
DECONTAMINATION SYSTEM
The decontamination is accomplished by immersion in a series of polypropylene vats sequenced to optimize the CORPEXÒ Process for this application. The sizes of the vats determined by the component sizes. The finned tubing was cut to 12.5 foot lengths for use in the dipping application. The tubes were capped before the first immersion to prevent liquid from entering the inner surfaces. The seven vats are 2 ft x 4 ft x 14 ft long with a total floodable volume of 838 gallons and a working volume of about 690 gallons.
Each tank is flat bottomed with no bottom drain. A bottom drain would have been preferred for ease of tank washouts, however the client was concerned about leakage and requested no drains. This resulted in the tanks having a liquid heel left after they were pumped down. The liquid heel was removed manually.
The entire system was enclosed by a two foot berm with the interior being water-proofed with a butyl rubber lining. The destruction tank was located in a completely separate berm. The work area=s ventilation system was aligned to take a suction from a cover placed on the destruction tank with the exhaust directed outside the facility. The destruction process releases minute quantities of some noxious smelling gases. Gas chromatographic analysis of the gases from the exhaust showed no significant percentage (< 1 ppm) of any regulated airborne substance.
A separate holding tank for the CORPEXÒ -921 was installed to allow for changeout of the chemistry without delaying decontamination. The CORPEXÒ -921 tanks were piped separately from the other processing tanks to prevent any possible valve alignment errors resulting in the mixing of the chemicals.
The particular system optimizes the use of the CORPEXÒ -921 so that it is used to depletion. Cleaning time did not increase noticeably as the chemical was depleted and the decontamination solution radioactivity levels did not become a limiting factor. Much of the activity was removed by the CORPEXÒ -934 tank with ultrasonics, although not enough to allow free release of the steel. Cleanout of this tank produced a large quantity of sludge which was more radioactive than that produced by the oxidation step.
Materials of construction for the CORPEXÒ Process equipment are typically welded 316 Stainless Steel and chemically resistant materials such as TeflonÒ . Polypropylene tanks and piping are adequate as CORPEXÒ -921 is compatible with it; however, the temperature must be kept below 80°C when using this material.
The full decontamination system consists of the following components:
Table I.Full Decontamination System

MATERIALS HANDLING
To assist with handling the tubes, a mono-rail system with three separately operated electrically powered hoists was installed over the centerline of the process line of decontamination vats. The rated capacity of each hoist was 1 metric ton.
DECONTAMINATION PROCESS
Typically, fourteen 12.5 foot finned tubes were loaded on a rack which was dipped successively in chemicals and rinses to decontaminate. The complete process consisted of seven steps:
DECONTAMINATION RESULTS
In total, 103 metric tons of contaminated carbon steel finned tubing was processed by Jordan Engineering using the CORPEXÒ Nuclear Decon Process. After a single pass, the bulk of the tubes were surveyed and found to have activity below the limit for free release (<0.2 Bq/gram including the 2X safety factor). A few tubes required a second pass to achieve free release, usually due to a hot spot. The location within the steam generator for the various tubes was not recorded during dismantlement, but it is assumed that these hard to decontaminate tubes were located at the hot (top) part of the steam generator. Approximately 3 tons of tubes (~150 12.5' sections) were not free released after the chemical process. These tubes will be cut to remove the contaminated areas and further reduce the amount of tubing that cannot be free released.
At the end of the processing, 99.5% of the tubing processed was able to be free released. It is estimated that the final weight of tubes that will not be free released will be less than 0.5 tons.
During the decontamination, the depletion of the CORPEXÒ -921 batch was monitored by a colorimetric titration test which measured free chelant capacity and also by the amount of iron in solution. The colorimetric titration test proved to be difficult to use due to the highly colored solutions formed by iron and cobalt complexation. The iron content as a function of tubes processed demonstrates that the rate of complexation (decontamination) is not affected by the depletion of the CORPEXÒ -921.
WASTE MINIMIZATION FROM CHEMICAL PROCESS
The chelants in CORPEXÒ -921 are destroyed by treatment with a chemical oxidizing agent such as sodium hypochlorite (the active ingredient in household bleach). As the chelant is destroyed, the metal ions are precipitated as their oxides and hydroxides. The primary products of the oxidation reaction are nitrogen, carbon dioxide and water. The precipitate may be separated from the solution by settling or filtration. The remaining liquid has a residual chelant concentration of less than 1000 ppm (<0.1 % by weight).
After oxidation of the chelants, the initial process used required raising the pH to 13.5 to complete the precipitation of the metal hydroxides. The precipitate was of very small particle size on the order of 2 m . Since the tanks were flat bottomed with no drains, the filtrate could not be removed in a economical manner. Manually decanting the liquid was time consuming and difficult to achieve since any disturbance caused the precipitate to go back into suspension. The value of the volume reduction resulting from this decanting was offset by the time required to complete the process.
Hunter and Associates (UK) Ltd, who provided a local chemical technology service throughout the project were responsible for introducing their novel HAPL process to precipitate and filter the metal hydroxides resulting from the oxidation process. This is a patented process which allows the precipitation of the notoriously difficult hydrated iron oxide to be done in a more controlled manner, resulting in a more easily filtered solid product of low volume.
Filtration of the hot post-oxidation solution after it has been treated by the HAPL process was performed using a 40 chamber, 250 liter filter press. The filter cake was easily washed on the filter to reduce chloride levels in the solid. The compact solid was then discharged from the filter press and was dry enough to be crumbled by hand. This form did not require further stabilization for disposal, further reducing waste volumes.
The use of the HAPL precipitation step minimized the total waste due to:
WASTE VOLUME REDUCTION RESULTS
Waste volumes generated by the free release of over 103 metric tons of finned tubing:
Table II. Waste Volume Reduction Results
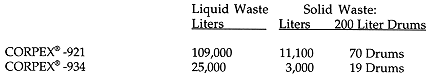
The drums of waste were not completely filled in case a decision to stabilize the waste was made at a later date. Each drum contained 68 kilograms of waste for a total of 6.1 metric tons. Therefore 103 metric tons of carbon steel was weight reduced by a factor of 17. All of the liquid waste was of sufficiently low activity and low dispersed solids that it was released to the plant waste treatment system. If the decontamination had not been performed to free release standards, the finned tubing would have required 6 isotainers at 20 tons per isotainer. Each isotainer has a total volume of 19.5 m3, so the total waste volume with no decontamination would have been 117 m3. After decontamination, the total volume of solid waste was 17.8 m3, for a volume reduction factor of 6.6. Based on the learnings from this first steam generator finned tubing decontamination project (including the addition of the HAPL precipitation step), the solid waste volume would be even lower on future projects of this nature.
SUMMARY
Using the CORPEXÒ Nuclear Decon Process, Jordan Engineering was able to decontaminate 99.5% of steam generator finned tubing from the Berkeley Nuclear Power Station to levels which permitted free release.