ESTIMATION OF AQUEOUS CHEMISTRY
AT A CRITICALITY SAFE SELECTIVE
ISSOLUTION OF THE AFTER-ACCIDENT
FUEL-CONTAINING MASSES
INSIDE OF CHERNOBYL UNIT-4 SHELTER
Sinitsyn V.A., Tokarevsky V.V., Khodorivsky M.S., Kulik D.A., Abramis
A.Ya.
R&D Centre "META" of State Company "Technocentre"
Kyiv, Ukraine
ABSTRACT
Chemical dissolution of fuel-containing materials is suggested as a feasible approach to removal of the highly radioactive nuclear fuel left-overs from the Unit-4 Shelter, which is a crucial step towards transforming of this object into an environmentally safe site. To estimate chemical composition of aqueous solution to be used for criticality-safe selective dissolution of fuel-containing masses, physical-chemical calculations were performed using "Selektor-A" code (Gibbs energy minimization method). Modeling results demonstrate that the moderately-alkaline sodium carbonate solutions could maintain concentrations of leachates (first of all, uranium) sufficient for the effective extraction. Moreover, solutions of this composition are not chemically reactive with respect to concrete and other materials thus providing structural stability of the Shelter. Such solutions can also contain dissolved boron at a level sufficient for nuclear safety. However, calculations show that interactions between solution and different solids of complex composition could cause precipitation of calcium borates or even boron-silicate compounds. Eventually, such processes will decrease dissolved boron concentration, hence additional experimental investigations and engineering developments should be carried out to find the best solution of the related technological problems.
INTRODUCTION
The Shelter ("Sarcophagus") was erected in 1986 over the destroyed Unit-4 of the Chernobyl nuclear power plant to isolate the after-accident radioactive materials from the environment. High levels of radioactivity of these materials were caused by the nuclear fuel left-overs (NFL) which were estimated to comprise up to 90% of the primary amount of fuel (before April 26, 1986). Presently, removal of NFL from Unit-4 Shelter is regarded as the crucial step towards transforming the object into an environmentally safe site [1]. NFLs inside of the Shelter are presented by fuel-containing materials (FCM), which, according to [1], are generally defined as:
FCM referred to as groups 1, 2 and 3 consist of uranium oxide as major component, whereas FCM of groups 4, 5 and 6 are much more chemically complex.
Lava-type FCM have been studied in detail [ 2,3]. The matrix of lava-like materials is a silicate glass (>65 wt.% of SiO2), containing K, Ca, Mg, Al, U, Zr impurities with no more than 3-4% of each element. In the lava matrix, there are numerous inclusions of crystalline phases, such as uranium oxides containing Zr impurity, crystals of high uranium zircon (HUZ), and Zr-U-O phases of uncertain composition, crystal structure and oxidation state of the metals. The HUZ contains up to 10 - 11% of uranium [3].
Dispersed FCM exist in the form of "hot" particles up to 10-40 m size. 20% of dust is graphite. Composition of other particles varies depending on the content of UO2 and different components of the structural materials (Fe, Zr, Si, Al, Ca etc.) up to pure UO2 [2,10]. Part of nuclear fuel containing dust results from disintegration of lava-like material and is chemically similar to the lava matrix [4].
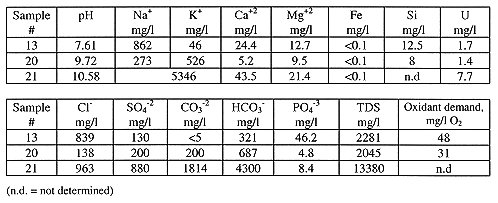
Nuclear fuel materials in solution are represented by highly radioactive waters (aqueous solutions) inside of the Shelter. Abramis et al. [5] performed a detailed study of the Shelter water composition. The waters are found to be mainly of chloride-bicarbonate, potassium-sodium, alkaline (pH 7.6 to 10.6) type, mildly reducing (in situ determined values of Eh are -83 to -112 mV) with total dissolved solids (TDS) 1 to 13 g/l and uranium concentration up to 40 mg/l. High values of the oxidant demand (permanganate addition) point to a high amount of dissolved organic compounds present, although their specific composition is still not known. Composition of three typical water samples is given in Table I.
Table I. Composition of Typical Shelter Waters

The aqueous solutions cause redistribution of nuclear fuel components from solid FCM. In particular, secondary uranium solid phases can form by evaporization of the Shelter water [6]. In previous work [7], the following newly formed uranium minerals are described: Na4(UO2)(CO3)3 . nH2O, UO2CO3 and UO3 . 2H2O.
Evidently, different techniques will be required for the removal of different FCM types from the Chernobyl Unit-4 Shelter. In this work, materials of groups 2, 3 and 4 are considered, for which no clear technological approach is available so far because of specific properties of these materials. In particular, complex spatial distribution, variety of forms and dimensions of accumulates, as well as irregular intermixing of highly radioactive substances with structural materials inside "Shelter", pose extreme problems in application of the mechanical destruction as part of the effective and safe removal technologies.
Leaching Technological AL Approach
Investigations on chemical processes inside of the "Sarcophagus" by means of analytical studies [5] and geochemical modeling [6,8] have demonstrated that the sodium-potassium-hydrocarbonate moderately-alkaline "Shelter waters" can selectively leach significant quantity of uranium together with other radionuclides from the FCM, while Zr, Fe, Ca, Mg are rather insoluble in such waters. Hence, these results suggest an applicability of experience accumulated in ore-mining industry and hydrometallurgy [9] to development of the technology of selective extraction of nuclear fuel components from the after-accident highly-radioactive materials.
The leaching technology based on selective dissolution of ore by chemical reagents followed by the removal of productive solutions from the reaction zone. In situ leaching is widely used as efficient method of exploration and processing of uranium ores. Depending on chemical composition of reagent, acidic and carbonate solution methods are used in uranium extraction. The choice of reagent is defined by the actual structure and mineralogy of the ore [9]. Under conditions of the Unit-4 Shelter, the specific requirements apply to the extracting aqueous solutions:
Given the appropriate conditions, it may be expected that radionuclides uranium-235, plutonium-239, 241 and americium-241 located inside of the Sarcophagus would initiate chain reaction with noticeable energy release. As well known, the energy release rate and possible negative consequences of such process in the system containing fissile nuclides depends on the value of so-called neutron multiplicity coefficient Keff determined as the ratio of number of neutrons in two subsequent generations. For Keff < 1, chain reaction is impossible and such systems is called undercritical. For Keff = 1, the system is critical with constant energy release (this is nuclear reaction). For Keff >1, the energy release grows exponentially and the system is called overcritical.
Permanent monitoring during several years has shown that FCMs located inside of the Sarcophagus were in undercritical state. Neutron energy spectrum in the multiplicative media, especially the number of thermal neutrons with velocities equal to the velocity of thermal movement, has the strongest influence on the Keff value. The number of thermal neutrons depends on the properties of multiplicative media: (i) slow-down ability ( moderation ); (ii) absorption ability (neutron absorption).
Moderation is governed by presence of such elements as hydrogen, beryllium and carbon in the FCM composition. Neutron absorption, on the other hand, depends on the concentration of strong absorbing nuclides like boron, gadolinium, hafnium and some others. Therefore, emergence of water in the pile-up of FCM may cause increase of Keff due to perfect moderative property of water. This explains the nuclear criticality danger of accidental water addition into the Sarcophagus. At the same time, presence of neutron absorbing nuclides in the water causes an opposite effect - decrease of Keff. But, the above requirements (1) and (2) must not be violated by introducing any neutron-capturing compounds into the aqueous solutions.
Physical-chemical calculations have been performed to estimate chemical composition of aqueous solution that corresponds to demands listed above and could be used for criticality-safe selective dissolution of after-accident FCMs inside of the Chernobyl Unit-4 Shelter.
AQUEOUS CHEMISTRY ESTIMATION
Modeling approach
Calculations were performed using a method of Gibbs energy minimization (GEM) implemented in Selektor-A code for PC [11] based on an Interior Points Method (IPM) convex programming algorithm [12]. The code finds explicit (meta)stable phase-component speciation x (including aqueous, gas and solid-solution components); values of chemical potentials of stoichiometric units u; pH, Eh and pP(gases). Input parameters are temperature T, pressure P, bulk chemical composition of the whole system b, partial molal (molar) Gibbs energies for all species g0TP, and set of equations for non-ideality models q (if appropriate). One aqueous electrolyte, one gas mixture, and any number of crystalline and dispersed (single- or multi-component) solid phases can be included in the system. This actually means that the solubility, gas-aqueous and redox equilibria in complex solid solution - aqueous solution (SSAS) systems can be calculated by GEM in one run, provided that all input thermodynamic data are available at TP region of interest.
In this study, model calculations were performed for ambient conditions (25oC, 1 bar) in the system Na-Ca-Mg-Fe-Al-Si-U-C-S-B-H-O. Activity coefficients of individual aqueous species were calculated using the extended Debye-Hueckel equation, with common third parameter set to 0.064. Solid solutions and gas phases were taken as ideal mixtures as first approximation. Total chemical composition of the modeled systems was specified using quantities of solid salts and minerals per 1 kg of water.
Thermodynamic database
An extensive consistent set of thermodynamic data was used in calculations which includes more than 200 species. The standard values of Gibbs energies of formation (D Go298.15 ) for gas, aqueous and solid species were taken from the built-in thermodynamic database of Selektor-A code. The structure and tools of this database are described in [8,11].
Aqueous solution is represented with a comprehensive set of aqueous species for ion-association model, including dissolved gases and solutes for the system Na-Ca-Mg-Si-Fe-Al-U-B-C-S-H-O. Thermodynamic properties of the most of species were listed in the previous work [13]. Sources of thermodynamic values for uranium complexes, as well as carbonate complexes of Na, Ca, Mg and Fe are cited in [6]. D Go298.15 values for aqueous species of boron are presented in Table 2.
Gas phase is represented by an ideal mixture of ideal gases (CH4, CO, CO2, H2S, NH3, NO2, SO2, SO3, H2, N2, O2) which can cover a wide range of redox conditions. Thermodynamic properties of gases were taken from [14].
Minerals were included in calculations of the "solid-water-atmosphere" equilibria as single phases (kaolinite , gibbsite, quartz, brucite, amorphous SiO2 [14], serpentine, hydrogoethite, hydromagnetite, mackinawite, whitlockite, disordered dolomite [15-17], salts in the subsystem Na-K-Ca-Mg-CO2-SO3-H2O [14,16,17]). Uranium phases were represented by uranium oxides, Na2UO4, Na2U2O7, Na3UO4, NaUO3, b -UO2(OH)2, UO2CO3 and Na4UO2(CO3)3 [18]. D Go298.15 values for the boron-containing solids are listed in Table 2.
Thermodynamic model of fully hydrated Portland cements is described in [6,13]. It includes single-component (ettringite Ettr - Ca6Al2S3O12(OH)12 , hydrotalcite Htc - Mg4Al2O7(H2O)10, portlandite Port - Ca(OH)2,cr) and solid-solutions phases (CSH1 and CSH2 phases of amorphous calcium silicate hydrogel, and "hydrogarnet" solid solution Hgr). Thermodynamic models of CSH phases have been developed in [13,19]. For the purposes of this work, ideal solid solution models with sodium end-members [13] were used, namely: CSH1 solid solution with SiO2, Ca0.9H1.8SiO3.8 and CaH2SiO4"NaOH end-members; and CSH2 with Ca0.9H1.8SiO3.8, Ca1.7H3.4SiO5.4 - 4H2O and CaH2SiO4 "NaOH end-members. The Hgr solid solution is represented by three components: Ca3Al2O6 - 6H2O, Ca3Fe2O6 - 6H2O and Ca3Al2Si3O12. Thermodynamic properties for the listed fully hydrated Portland cement single-component phases and end-members were taken from [13].
Table II. D Go298.15 of Boron-Containing Species and Solids Used in Calculations

Model calculations
Interaction of aqueous solution with Portland cements was modeled to estimate stability of the main construction materials of Shelter with respect to possible reagents of different composition. Since the real composition of concrete in the interior of Chernobyl Shelter is unknown, the following composition of Portland cements was used for the model (wt.%): CaO - 65, SiO2 -23, Al2O3 -7, Fe2O3, - 3, MgO - 1.5, SO3 - 0.5. The aqueous ingredients were real Shelter water compositions equilibrated with air [5,6] as well as acid (H2SO4) or soda concentrations proposed for the in situ leaching of uranium from the ores [9]. Modeling was performed for solid/water ratios ranging from 10-7 to 1. At each point, the equilibrium phase assemblage, quantities of phases and compositions of solutions were determined. To investigate the behaviour of dissolved boron in an interaction pathway, 200g of H3BO3 per 1000g H2O was added to model system. Previous calculations [6] have shown that interaction of concrete with carbonate-bicarbonate Shelter water results in carbonatisation of high calcium cement phases, accompanied with a decrease in dissolved carbonate. As a consequence, secondary uranium minerals will precipitate, and Sraq concentration will drop as a result of (Ca,Sr)CO3 formation. Also, Mgaq concentration in the Shelter water is close to the concrete saturation values. Siaq , Alaq exceed, while Caaq does not approach the concrete saturation values. Evidently, this can lead to Ca leaching via portlandite dissolution, although the aluminosilicate phases of the concrete are sufficiently stable in this environment. These results are confirmed by observations of real concrete after a 10-year period of interaction with water inside of the Shelter. Corrosion of the concrete was never observed to deteriorate the structural stability of the object. The Shelter waters (Table 1) and carbonate reagents used for in situ uranium leaching [9] are comparable with respect to alkalinity and ionic composition. Although the leaching reagents are 2-5 times more concentrated, the calculated models show that during interaction between Portland cement and typical soda reagent, the concretes are stable materials. Therefore, the carbonate method should be regarded as preferable compared to the acidic one, especially taking into account that recommended concentration of sulphuric acid in reagents [9] well exceed 0.2 g/l and would cause sulphate corrosion of Portland cement materials [25].
Fig. 1 demonstrates total dissolved boron (Baq) concentration at equilibrium with Portland cement, soda aqueous solution (0.8m Na2CO3 + 0.25m NaHCO3) and 200g H3BO3 as function of cement/ water mass ratio. At log (solid/water) up to -2, where the phases of ordinary Portland cement (OPC) are not stable and only secondary minerals (SM) are present, such as ferrihydrite, kaolinite and Ca, Mg carbonates. Baq is controlled by sodium (Bx) and calcium (HB) borates. In the narrow solid/ water range, danburite (Db) is stable. At equilibrium with borax (Bx), Baq concentration is ~8 g/l, is decreasing to ~5 g/l with Hb and Db precipitation. After the OPC phases become stable, datolite is formed, accompanied with the sharp drop of Baq (< 0.1 mg/l).
Interaction of aqueous solution with uranium minerals was modeled to estimate the influence of reagent composition on uranium extraction performance. Recently, interaction of uranium oxides and high uranium zircon (HUZ) with aqueous solutions of Unit-4 Shelter was modeled [6,8]. These uranium phases represent some important types of FCM, such as the core fragments or pieces of nuclear fuel elements, dispersed fuel and crystalline inclusions in the matrix of lava-type FCM. Results [6,8] show that solid UO2 phases control U solubility in carbonate-bicarbonate Shelter water at Eh < 0; at oxic conditions, uranium oxide solubility in such waters can reach values up to 10-1 molal (ca. 20 g/l), although measured concentrations of Uaq (see Table 1) are still much lower than the limiting solubilities of both UO3 and UO2CO3. However, measured Uaq seems to be controlled by the Na-autunite Na2(UO2)2(PO4)2 (sample #13) or Na4(UO2)2(CO3)3 phase (sample #21) , which points to the significance of secondary minerals for redistribution of uranium in the "geochemical" system of Shelter. HUZ interaction with both sample #13, and sample #21 water in a wide range of redox conditions results in leaching of uranium from this metastable phase. Zraq concentration is considerably less than Uaq concentration, even under reducing conditions [6]. It is evident that the phase assemblage of the HUZ decomposition products should be dependent at the degree of U leaching. For high leaching (for example, at oxidizing conditions), HUZ decomposition can trigger precipitation of secondary U-minerals. Therefore, alkaline Shelter waters must be regarded as chemically reactive with respect to uranium-bearing FCM. Real Uaq concentration in the waters could be explained by precipitation of moderately-soluble secondary phosphates and carbonates of U(VI), as well as by mildly reducing conditions (in situ determined values of Eh are -83 to -112 mV) of aqueous media at the surface of U(IV) containing materials. Figs.2 and 3 demonstrate U oxide and H3BO3 solubilities in the carbonate reagents depending on soda concentration and redox conditions. An increase of Na2CO3 above 1 mol/kg (~ 10 %) leads to a drop of Uaq concentration (Fig.2), caused by formation of weakly soluble Na-UO3 phases. As the solution becomes more concentrated, Baq concentration also decreases, but not lover than 5 g/l. It is well known that maximum Uaq concentration is reached in oxidizing solutions. This fact is illustrated in Fig.3, where the redox-independent solubility of H3BO3 is also shown.
DISCUSSION
In situ leaching should be regarded as feasible approach to remove some varieties of FCM from Chernobyl Unit-4 Shelter. Vast experience accumulated in ore industry and hydrometallurgy is itself a sound basis for further development of the technology of selective extraction of uranium together with other radionuclides, taking into account specific conditions of the Sarcophagus. The carbonate leaching of uranium from the FCM is preferable compared to the acidic method because concrete corrosion by acidic reagents (such as sulphuric acid) endangers the structural stability of the object.
Typical carbonate reagents using for the in situ uranium leaching are chemically similar to mildly alkaline hydrocarbonate-carbonate waters already formed inside of the Sarcophagus as result of various natural processes and engineering activities. These waters contain dissolved uranium up to n - 10 mg/L and did not appreciably affected the concrete materials so far. Results of GEM physical-chemical modeling demonstrate that carbonate reagents can selectively leach uranium from FCM. Efficiency of uranium extraction can be adjusted by variation of solution chemistry (alkalinity, soda concentration) and redox conditions. Up to now, uranium inside Sarcophagus exists mainly as UIV-form which reduces the surface film of reacting water solution [5], therefore the usage of oxidants should be necessary to speed up the process. The calculations show also that carbonate reagents are not too aggressive media in respect to constructive materials and could contain dissolved boron much more than it is necessary to maintain the nuclear safety (at Keff<< 1) of leaching technology.
From previous [5,6] and present data one can conclude that mixing of chemically complex Shelter water with leaching reagents can lead to precipitation of weakly soluble solids such as autunite owing to elevated concentration of dissolved phosphate [5], as well as borates and boron-silicates of calcium (see Fig. 1.C). This, in principle, can decrease efficiency of uranium removal and dissolved boron concentration. Moreover, equilibration of uranium- and boron-bearing alkaline carbonate solutions with high calcium cement materials will result in the formation of weakly soluble minerals (Na2U2O7 [6], danburite, datolite - see Fig.1) on the concrete surfaces. Possible leachate losses of this kind need to be monitored, although coprecipitation of uranium with boron clearly will not increase criticality and nuclear danger.
The complications discussed above, however, do not comprise insuperable obstacles in the implementation of the in situ leaching approach for the removal of FCM. Additional experimental investigations and engineering developments should be conducted in order to work out the best solutions of the related technological problems.

Fig. 1. Modeled Equilibrium Total Dissolved Boron Concentration, H+ Activity (A) and Phase Assemblages (B,C) Formed as a Result of Interation of Portland Cement with the Soda Aqueous Solution (0.8m Na2CO3 + 0.25m NAHCO3) and 200g H3BO3 at 25° C, 1 bar.

Fig. 2. Equilibrium Values of Total Dissolved Uranium and Boron Concentrations, Activity of H+ and Eh in the system: 200 g H3BO3 + 1mole UO2 + 0.25m NaHCO3 + 1mole O2 + 1kg H20 in dependence on concentration at Na2CO3 at 25° C and 1 bar.
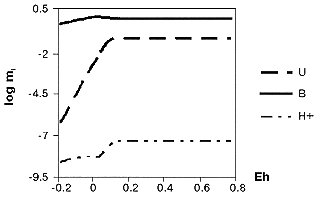
Fig. 3. Equilibrium Values of Total Dissolved Uranium and Boron Concentrations and Activity of H+ in Dependence on Redox Conditions for the System: 200 g H3BO3 + 0.25 mole NaHCO3 + 1 mole UO2 + 1 mole Na2CO3 + 1kg H2O at 25° C and 1 bar
REFERENCES
Chernobyl Unit-4 Shelter Implementation Plan (SIP), Tacis services DG IA, European Commission and US Department of Energy (1997).
B.BURAKOV, E.GALKIN, E.PAZUKHIN et al., Radiochim. Acta v.65 (1994).
A.KISELEV, A.NENAGLYADOV, A.SURIN et al., Preprint IAE, 5533/3, Moscow (1992).
A.S.VISHNEVSKY, A.G. GONTAR, I.E.KUZ`MINA et al. "Aerosol of the Encasement" Preprint of the Institute of superhard materials, NAS of Ukraine, Kyiv, 32 p. (1997).
A.ABRAMIS, M.KHODORIVSKI, V.SINITSYN et al., in Problems of Chernobyl Exclusion Zone N 6, Naukova Dumka Publishers, Kyiv (1997).
V.SINITSYN, D.KULIK, M.KHODORIVSKI et.al., Mat.Res.Soc.Symp.Proc. v.465 (1997).
A.VISHNEVSKII, I.KUZMINA, V.TKACH and V.TOKAREVSKY, in Problems of Chernobyl Exclusion Zone N 3, Naukova Dumka Publishers, Kyiv(1996).
D.KULIK, V.SINITSYN, M.KHODORIVSKI et al., NRC4 Conf. Extended Abstracts. (Eds: F.David, J.Krupa), St.Malo (1996).
V.MAMILOV, R.PETROV,G. SHUSHANIYA et al. "Exploration of Uranium by Methods of Underground Leaching," Atomizdat, Moscow (1980).
M.KHODORIVSKI, N.PROSKURA, V.SINITSYN and D.KULIK, Abstr.of 16th Met. of Intl. Mineral. Ass. Pisa (1994).
D.KULIK, S.DMITRIEVA, K.CHUDNENKO et al.,"Selektor-A Test-Version 3.1b for DOS. User's Manual (draft)," Brooklyn-Kyiv (1997).
I.KARPOV, K.CHUDNENKO and D.KULIK, Amer. J.Sci., v.297 (1997).
V.SINITSYN, D.KULIK, M. KHODORIVSKY, and I.KARPOV, Mat. Res. Soc. Symp. Proc. (1998) in press.
R.ROBIE and B.HEMINGWAY, U.S.Geol. Surv. Bull. 2141 (1995).
Y.MELNIK, "Genesis of Precambrian Banded Iron Formations," Naukova Dumka Publishers, Kyiv (1986).
Y.KHARAKA, W.GUNTER, P.AGGARVAL et al., U.S.Geol. Surv. Water-res.Invest.Rep.88-42, Menlo Park,CA (1988).
J.BALL and D.NORDSTROM, U.S.Geol.Surv.Open-File Rep. 91-183, Menlo Park,CA(1992).
I.GRENTHE, J.FUGER, R.J.M.KONINGS et al," Chemical Thermodynamics of Uranium," North-Holland, Amsterdam et al. (1992).
D.KULIK, V.SINITSYN, and I.KARPOV, Mat.Res.Soc.Symp.Proc.(1998) in press.
J.JOHNSON, E.OELKERS, and H.HELGESON, Comput. Geosci. v.18 (1992).
G.NAUMOV, B.RYZHENKO and I..KHODAKOVSKY, "Handbook of Thermodynamic Values," Atomizdat, Moscow (1971).
F.MILLERO and D.SCHREIBER, Am.J.Sci. v.282 (1982).
"Thermochemical Constants of Compounds," v.V , Ed: V Glushko, Acad.Sci.USSR Publ. Moscow (1971).
"Analytical chemistry of elements. Boron," Ed: A.Vinogradov, Nauka, Moscow (1964).
V.MOSKVIN, "Corrosion of concrete," Gosstroyizdat, Moscow (1952).
BACK