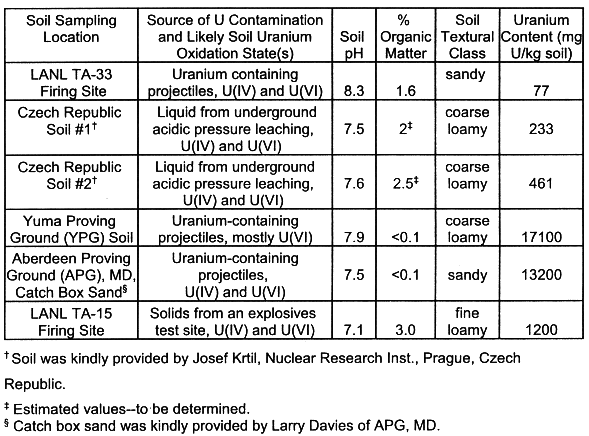
Table I. Characteristics of soils selected for the acid and base
leach comparison.
Martine C. Duff, Caroline F. V. Mason, J. A. Musgrave
Chemical Sciences and Technology Division
Los Alamos National Laboratory
(LANL)
Los Alamos, NM 87545
Frank Dunfrund
U.S. Army
Yuma Proving Ground (YPG)
Yuma, AZ 85365
ABSTRACT
Over the past few years, several different leaching schemes have been developed for the removal of uranium from contaminated media such as soil. Studies using sulfuric acid (H2SO4) and carbonate leach solutions (with or without an oxidative pretreatment) have been proposed. These solutions have been used on environmental sites with varying degrees of success. However, at few of these sites have both acid and base leachings been evaluated using the same set of criteria. The lack of comparative studies on acid and base leaches has created confusion over the relative merits of the two approaches. A comparison of selected acid and base leaches with several U-contaminated soils will be discussed. Discussion will be given with supporting data on relative U removal efficiencies for each leach solution and the other leached elemental concentrations. Conclusions of our research will assist in the decision-making processes concerned with the remediation of U-contaminated media. Preliminary studies suggest the removal efficiency of soil U for each leach solution varies with the nature of the U contamination in the soil. It is likely the form(s) of soil U such as reduced species, absorbed species and mineral forms influence the solubilization of U by the leaching agents.
INTRODUCTION
In the past and present, U has generally been mined from ore with one of two U solubilization methods: sulfuric acid and carbonate leach (MERRITT, 1971). These solutions are sometimes preceded by an oxidation with peroxide, O2 or air. In sulfuric acid, U forms several U-sulfate complexes which are most soluble at low pH. A few of these reactions are:
SO42- + U4+ <----> USO42+ ,
2 SO42- + U4+ <----> U(SO4)2o,
Fe2(SO4)3(s) + UO2(s) <----> UO2SO4o + 2 FeSO4(s),
2 SO42- + UO22+ <----> UO2(SO4)22-, and
3 SO42- + UO22+ <----> UO2(SO4)34-.
Carbonate leaching involves the formation of various highly soluble U-carbonate complexes which are not likely to absorb to negatively charged soil constituents (DUFF and AMRHEIN, 1996). Some of the key reactions in this process are:
CO32- + UO22+ <----> UO2CO3o,
2 CO32- + UO22+ <----> UO2(CO3)22-,
3 CO32- + UO22+ <----> UO2(CO3)34-, and
6 CO32- + 3 UO22+ <----> (UO2)3(CO3)66- (CLARK et al., 1996).
MATERIALS AND METHODS
Several U-contaminated soils (Table I) were chosen for leaching with the selected leaching solutions described in Table II. Soil-water batch mixtures were equilibrated in 50 mL polycarbonate centrifuge tubes for three-48 hour periods. Batch soil to water ratios were 1:20 and the equilibrations were done in triplicate. At the end of each equilibration, the samples were centrifuged at 17300 r.c.f. for 15 minutes and the supernatants were decanted. The pH of the solutions was determined. The solutions were diluted and prepared for the determination of U and other soluble elements (Mn, Fe, Si, Pb, Cr and Ca). Dissolved U was determined with a kinetic phospholuminescence analyzer (Chemcheck, WA) and soluble ions were determined by ICP-AES (Leeman Instruments, NY). Unleached soils were acid digested to determine total U using a modified method of LIM and JACKSON (1983) in which HF acid is not used. [The HF forms volatile compounds with U at elevated temperatures--resulting in the loss of U during the soil digest (WEIGEL, 1986; LEE and WU, 1991)].
Table I. Characteristics of soils selected for the acid and base
leach comparison.

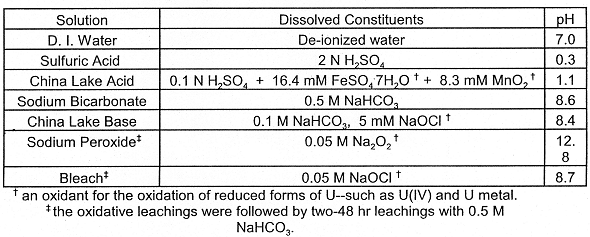
Table II. Leaching solutions used in this study. For simplicity,
these solutions will be referred to in this paper by the names in the far left
column of the table.
RESULTS
Uranium Removal by Leaching Solutions:
Six of the seven leaching solutions were selected because they have been used to remove U from soil and from contaminated surfaces. The Sulfuric Acid solution was used by Oak Ridge National Laboratory (USID, 1992) for preliminary laboratory studies on Fernald, OH soils. Uranium contaminated soil from China Lake Naval Weapons Center was leached on site with the solutions labeled China Lake Acid and China Lake Base (Wenstrand and Greene, 1993). The China Lake Acid contains reduced Mn2+ and oxidized Fe3+ which react to form Mn4+ and zero valent iron (Fe0), which has been shown to be a good oxidizing agent for reduced U species (Wenstrand and Greene, 1993). LANL has used the Sodium Bicarbonate, Sodium Peroxide and Bleach solutions for Firing Site soil and the surface decontamination of explosion debris (unpublished research at LANL by Nan Sauer and Brandy Duran, 1996). The Sodium Peroxide and Bleach solutions are oxidizing agents which can convert reduced forms of U to more soluble oxidized forms such as U(VI).
TA-33 Firing Soil: The Bleach solution (followed by the Sodium Bicarbonate solution) removed 70% of the total soil U. The Sulfuric Acid, China Lake Acid, Sodium Bicarbonate and China Lake Base solutions (listed Table II) leached nearly the same cumulative quantity of U from the TA-33 soil (Tables III and IV). Additionally, most of the U was leached within the first 48 hour equilibration by the four solutions. The U in the TA-33 Soil (Tables III and IV) was efficiently leached with Sodium Bicarbonate after the soil was equilibrated with the oxidants (Sodium Peroxide and Bleach solutions). Because of the greater quantity of U leached after the oxidation, it is likely this soil contains reduced U species (such as uraninite, UO2(s)).
Table III. Leached uranium (mg kg-1 Soil). The oxidative
leachings (Sodium Peroxide and Bleach) were followed by two-48 hr leachings with
0.5 M NaHCO3.
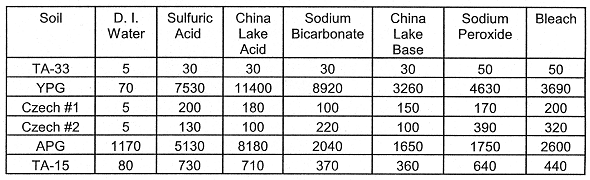
Table IV. Percent U leached. The oxidative leachings (Sodium
Peroxide and Bleach) were followed by two-48 hr leachings with 0.5 M NaHCO3.
Values in bold type indicate the greatest leaching efficiency for each row of
the table.
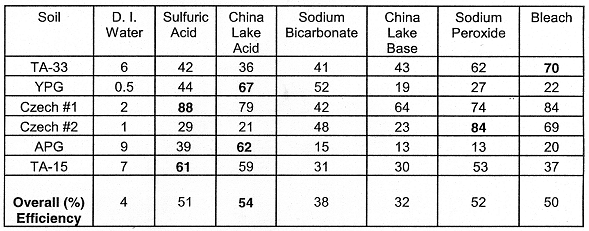
Czech Republic Soils #1 and #2: For the Czech Republic #1 (Cz. #1) soil, the Sulfuric Acid and Bleach leaching solutions solubilized more U than the other leaching solutions (Tables IIIand IV). As for the Cz. #2 (Cz. #2) soil, the leachings with Sodium Peroxide and Bleach solutions (followed by the Sodium Bicarbonate) solubilized more U than the other solutions (Tables III and IV). Hence, the Cz. #2 soil probably contains a significant quantity of reduced U species which are oxidized in Sodium Peroxide and Bleach.
The Czech Republic soils are highly organic and the soil U is likely to be associated with the organic matter (OM) and/or adsorbed to the soil surface. The high pH of the oxidizing solutions may dissolve soil OM which is soluble at high pH. However, the pH of the Bleach solutions after reaction with the Czech soils decreased from 8.7 to 6.0. This was not observed with other soils or leaching solutions used in this study.
The low pH of the Sulfuric Acid and China Lake Acid solutions (Table II) may facilitate the dissociation of U associated with OM or the soil surface--resulting in the formation of dissolved U(IV)- and U(VI)-sulfate species. A low pH of the acidic leaching solutions protonates soil surface sites and may displace positively charged adsorbed U species from the soil surface.
YPG Soil: For all of the leach solutions equilibrated with the YPG soil, the China Lake Acid solution removed the most U (Tables III and IV). The oxidation state of U solids in this soil is most likely U(VI)--based on XRD analyses of the highly visible U (schoepite) solids which were found in the YPG soil (MASON et al., 1996). The Sodium Bicarbonate solution removed nearly as much U (59% of the total U) as the China Lake Acid solution (67% of the total soil U).
APG CB Sand: The acidic leaching solutions were most efficient at removing U from the APG sand (Tables III and IV). The first 48-hours leaching was very effective at removing the U. The basic and oxidizing leaching solutions were comparable with respect to their removal efficiency of U from the sand.
LANL TA-15 Soil: As with the APG sand, the two acidic solutions were most effective at removing U from the soil (Tables III and IV). The Sodium Peroxide/Bicarbonate leaching was nearly as efficient at removing U from the TA-15 soil as the acidic solutions (53% for the sodium bicarbonate versus 59 and 61% for the two acid solutions).
Additional Elements in Solutions After Leaching:
The following elements were measured in the leaching solutions because elevated solution concentrations of these elements (such as Mn, Fe, Si and Ca) are indicative of soil weathering. Lead and chromium were examined because they are toxic metals which may complicate the safe disposal of leachate solutions because their presence makes the leachate a mixed waste.
Manganese (Mn): Manganese is a common soil constituent and is known to be highly soluble under acidic conditions (pH below 4). In soil, Mn often exists in the form of oxides, phosphates, carbonate and as a minor component of complex soil silicates. At low pH, the soil becomes chemically altered in the presence of excess hydrogen ion (H+)--which promotes the hydrolysis of Mn in the leachate solution. The acid solutions leached more Mn from the soil than the other leaching agents (Table V). The increased quantity of Mn in the acidic leaching solutions is indicative of soil weathering. Some likely components of soil weathering are a decrease in soil particle size, increase in soil particle surface area, changes in cation exchange capacity and changes in soil mineralogy.
Table V. Leached Mn (mg kg-1 Soil). The oxidative
leachings (Sodium Peroxide and Bleach) were followed by two-48 hr leachings with
0.5 M NaHCO3. The China Lake Acid contained Mn. Negative values
resulted when the amount of Mn added was subtracted from the quantity of Mn
measured in the China Lake Acid solution after equilibration with soil.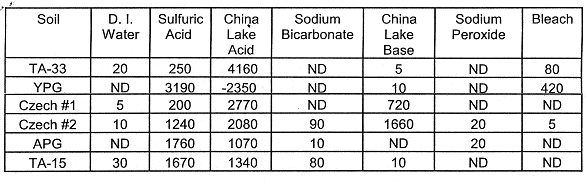
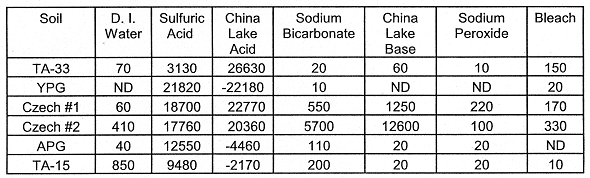
Iron (Fe): Iron, like Mn is also a common soil constituent. Its solubility is greatest at low and high pH (pH<3 and pH>10). Increases in soluble Fe may be indicative of soil weathering. In soil, Fe often exists as oxides, phosphates, silicates and to some degree, carbonates. As with Mn (above), the acidic solutions leached more Fe from the soils than the basic solutions. Two leaching agents, the Sodium Peroxide and China Lake Base solutions leached appreciable quantities of Fe from two soils (the YPG and the CZ. #2) such as the (Table VI). However, both acidic solutions appeared to have the greatest effect of leaching soil Fe.
Table VI. Leached Fe (mg kg-1 Soil). The oxidative
leachings (Sodium Peroxide and Bleach) were followed by two-48 hr leachings with
0.5 M NaHCO3. The China Lake Acid contained Fe. Negative values
resulted when the amount of Fe added was subtracted from the quantity of Fe
measured in the China Lake Acid solution after equilibration with soil.
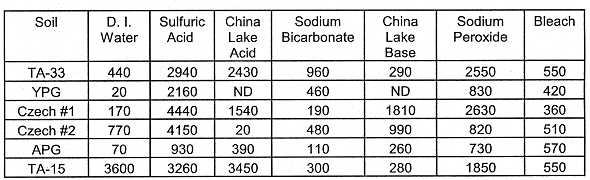
Silicon (Si): Silicon is soluble in high and low pH solutions (pH<2 and pH>10). Silica may exist as an oxide such as quartz (SiO2) or with complex soil silicates such as vermiculite (Mg0.3(Mg,Fe3+,Al)3(Si3Al)O10(OH)2). Generally, Si was quite soluble in the acidic leaching solutions (Sulfuric Acid and China Lake Acid) and the Sodium Peroxide and China Lake Base solutions as compared to the Sodium Bicarbonate and the D. I. Water (Table VII). Silica was most soluble in the Sulfuric Acid leaching solution.
Table VII. Leached Si (mg kg-1 Soil). The oxidative
leachings (Sodium Peroxide and Bleach) were followed by two-48 hr leachings with
0.5 M NaHCO3.
Calcium (Ca): Calcium is a common constituent in soils which is likely to be solubilized when soil silicates (containing Ca) are dissolved in the presence of acid. Calcium may also be in the form of carbonates (calcite) and sulfates (gypsum, CaSO4.2H2O(s)). For the YPG soil, Ca was highly soluble in the presence of Sulfuric Acid (Table VIII). This is most likely due to the presence of calcium carbonate (calcite, CaCO3(s)) which dissolves in the presence of acid--releasing Ca and carbon dioxide (CO2(g)). Calcium was most soluble in the acid treatments--regardless of the soil used in the leaching.
Table VIII. Leached Ca (mg kg-1 Soil). The oxidative
leachings (Sodium Peroxide and Bleach) were followed by two-48 hr leachings with
0.5 M NaHCO3.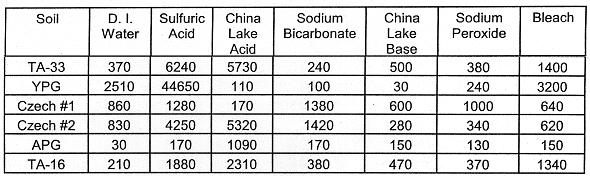
Lead (Pb): Lead is often a minor constituent in soils which are not contaminated. It is an EPA RCRA listed metal. In the soils leached with the acid solutions, Pb was more soluble--in comparison to the other leaching solutions (Table IX). For the Cz. #2 soil, Pb was appreciably leached by the Sodium Peroxide, Sodium Bicarbonate, and China Lake Base solutions. Lead was also leached by these three basic solutions in varying degrees for the APG and TA-15 soils. However, leaching of Pb from the soils was most evident in the acidic solutions.
Table IX. Leached Pb (mg kg-1 Soil). The oxidative
leachings (Sodium Peroxide and Bleach) were followed by two-48 hr leachings with
0.5 M NaHCO3.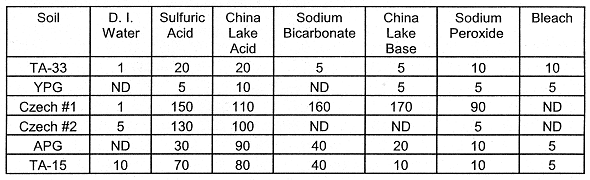
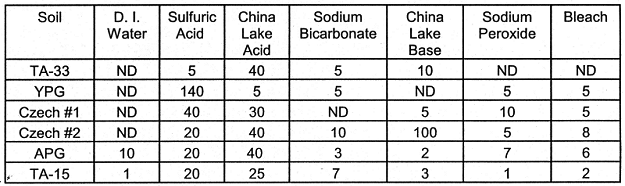
Chromium (Cr): Like Pb, Cr is usually a minor constituent in pristine soils. However, it too is a RCRA listed metal. For the most part, Cr was most soluble in the acid solutions (Table X). The oxidation state of the Cr was not determined. However in the environment, Cr(III) species are most likely to exist in acidic solutions whereas both Cr(III) and Cr(VI) species may be present in basic solutions (LOSI et al., 1994).
Table X. Leached Cr (mg kg-1 Soil). The oxidative
leachings (Sodium Peroxide and Bleach) were followed by two-48 hr leachings with
0.5 M NaHCO3.
DISCUSSION AND CONCLUSIONS:
For the soils, U was most efficiently leached by the China Lake Acid solution (Table IV). However, the detrimental effect of strongly acidic solutions such as the China Lake Acid on soil properties may outweigh the benefits of U removal. Strongly acidic solutions are likely to be detrimental to the physical and chemical aspects of soil structure and leach other toxic metals such as Pb and Cr. In the past, large scale leachings of U contaminated soils with strong acids have produced "slimes" which were found to be difficult to manage. The acidic U slimes were not filterable and contained a large concentration of U.
It is also apparent that each soil responds differently to the seven leaching solutions. Acidic and basic solutions can have substantial effects on soil--particularly, with respect to soil weathering and to the dissolution of potentially soluble species such as RCRA metals. It is possible that other hazardous metals not included in this study may be solubilized during leach remediation. It is concluded that approaches to soil and catch box sand remediation involving acid and base leaching should be made with caution.
These studies were conducted with soils which were contaminated with U by different processes. These processes are likely to influence the efficiency of the leaching agents. It is important to know the source of soil contamination by U, the oxidation state of the soil U and the soil properties.
ACKNOWLEDGMENTS
Many people have provided their technical expertise and assistance to this project. These people include Tate Hamilton, Wayne Taylor, Chuck Cotter, Carlos Garcia, Inés Triay, Laura Wolfsberg and Wayne Lemons all of Los Alamos.
REFERENCES