
Fig. 1. Simplified TWRS process
flowsheet.
David J. Swanberg and Phong M. Nguyen
SAIC
Joan Young
BDM
Waste Policy Institute Team
3250 Port of Benton
Boulevard
Richland, Washington 99352
ABSTRACT
The Clean Salt Process (CSP) can reclaim useful chemical products from alkaline tank waste at Hanford and other DOE sites substantially reducing the volume of waste for disposal. DOEs tank wastes are primarily sodium salt solutions once the radionuclides and HLW solids are removed. At Hanford, the waste contains an estimated 60,000 metric tons of sodium, most of which is recoverable as clean sodium nitrate salt. Tests with actual waste produced clean sodium nitrate (2 pCi/g residual radioactivity) that was released from the laboratory as non-radioactive material.
In the CSP, clean nitrate salts are selectively crystallized and separated from contaminated solution. Overall decontamination factors > 108 have been demonstrated with actual waste. For production implementation, a multi-stage CSP plant, placed downstream of cesium ion exchange, will produce clean salt suitable for commercial re-use. The preferred end use for the recovered salt is in the production of explosives.
RCRA regulations encourage recovery of useful products from waste. DOEs Office of Environment, Safety and Health has developed criteria for free release of materials with very low levels of residual radioactivity. Other possible end uses ofClean Salt do not require RCRA or DOE exemptions. These require additional processing but are still economically attractive.
The Clean Salt Process needs to be demonstrated at a larger scale. This will generate performance data and design parameters to support an accurate cost/benefit analysis. Radiation dose impact and ALARA analyses should be performed to establish de-minmus radioactivity levels for the recovered material.
INTRODUCTION
The purpose of this paper is to review the technical basis and regulatory requirements for the Clean Salt Process (CSP), to identify promising options for reuse of the CSP products, and to recommend a path forward.
Hanford underground storage tanks contain approximately 230 million liters of radioactive waste in 177 tanks. The waste is alkaline and consists of sludge, saltcake, and supernate (salt solution). Most of the radioactivity and hazardous constituents are found in the sludge. However, 85% of the waste (exclusive of water) consists of soluble sodium nitrate, nitrite, and hydroxide salts. Hanford's Tank Waste Remediation System (TWRS) project has developed a technical baseline for treatment of the waste (Fig. 1). Tank waste supernate and dissolved salt cake solutions will undergo Ion Exchange (IX) to remove soluble radionuclides followed by treatment and disposal as low-level waste (LLW). The sludge and radionuclides from IX will be vitrified in borosilicate glass and disposed of in the HLW repository.

Fig. 1. Simplified TWRS process
flowsheet.
Westinghouse Hanford Company and DOEs Office of Science and Technology (OST) developed the CSP as a method to significantly reduce the volume of waste for disposal and to recover useful products from the waste (1). Previous reports analyzed technical feasibility and regulatory requirements of the CSP (2-5). This paper compiles the most current information and analyses and recommends a path forward for the CSP.
TECHNOLOGY DESCRIPTION
The basis of the CSP is selective crystallization, a fundamental chemical process used routinely in industry to purify a wide spectrum of chemicals. To treat tank waste, the supernate is first acidified to pH 2. Sodium nitrate (and aluminum nitrate) crystals are then formed by concentrating or cooling the solution beyond the saturation point. The remaining liquid, containing most of the radioactivity, is drawn off and the crystals are washed with clean solution. Overall radionuclide decontamination factors (DFs) of up to 108 were observed in multi-stage (typically 4 to 5) experiments with Hanford waste (1). Single stage DFS were generally repeatable for different waste types and constant for several different radionuclides within a single waste type. Product salts with residual activity of 2 pCi/g were released from the lab as non-radioactive material. This compares to naturally occurring radioactivity in commercial grade sodium nitrate of about 0.3 pCi/g and is equal to measured specific activity of the human body.
For the plant scale process, the primary radionuclide of concern is cesium-137. For average Hanford feed, an overall DF of about 109 is needed to produce non-radioactive salts for commercial use. The production scale plant design calls for cesium removal followed by multi-stage crystallization to process average Hanford feed (2). A simplified diagram of this process is shown in Fig. 2.

Fig. 2. Clean salt process for Hanford tank waste.
Technical results to date indicate that CSP is a viable process that could be incorporated in the TWRS technical baseline. Engineering-scale data, however, are needed to understand whether the CSP will produce essentially non-radioactive salts on a production-scale. Parameters to test include the following:
Data should also be gathered from commercial industry on related processes such as sugar refining and fertilizer production.
ECONOMIC FEASIBILITY OF THE CLEAN SALT PROCESS
A preliminary cost estimate for the full-scale CSP process indicates that recovery and re-use of clean sodium nitrate from Hanford tank waste would be less expensive than vitrification and disposal as LLW. Construction and Operating costs for the CSP process applied to Hanford tank waste are estimated at $130 million (2). This would be offset by a $260 M credit for sale of recovered sodium nitrate as a commercial chemical product. A comparison of total costs for vitrification and application of the CSP appears in Table I. Vitrification and disposal costs were taken from the TWRS EIS Engineering Data Package for the Tri-Party Agreement (TPA) Alternative (6).
Table I Vitrification/CSP Cost Comparison for Hanford LLW
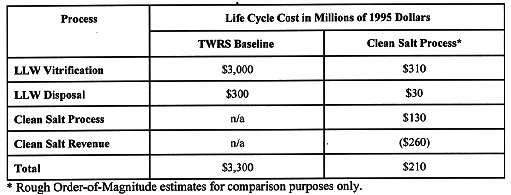
Most of the cost savings achieved through the use of the CSP are due to significant decrease in LLW volume. A new crystallization plant would eliminate the need for a large-scale LLW vitrification facility, as envisioned in Phase II of TWRS Privatization. With up to 90% waste volume reduction, the Phase I pilot scale vitrification processes may be adequate to process all Hanford LLW resulting in substantial capital and operating cost savings.
The cost estimates used for comparison have significant contingencies. However, the available data show that there is potential for cost savings on the order of billions of dollars which warrants serious consideration. Recommended next steps are to re-visit CSP costs based on better design definition, place the CSP and TWRS cost estimates on equivalent parametric bases, and perform sensitivity analyses to identify critical cost factors.
REGULATORY REQUIREMENTS
Hanford tank waste is regulated under the Resource Conservation and Recovery Act of 1976 (RCRA) Specific requirements depend directly upon the alternative selected for disposition of the recovered salt. If the salt is marketed as a commercial product, then the material and the recovery process are exempt from hazardous waste regulations. However, if the recovered salts are disposed of, used in a manner constituting disposal, or accumulated speculatively they would be considered waste and would continue to be regulated under RCRA.
Hanford tank wastes are listed and characteristic wastes subject to RCRA Land Disposal Restrictions (LDRs). Proper treatment of recovered salts would be required prior to placement on or in the land (e.g. in a landfill). If disposed of, or used in a manner constituting disposal, the salts would have to be de-listed through a petition process with the Environmental Protection Agency and the Washington State Department of Ecology.
Alternatively, listed wastes that pose minimal risk to human health and the environment may be allowed to exit RCRA under the Hazardous Waste Identification Rule (HWIR). Under this rule, concentration-based limits for listed waste constituents are derived through a global risk assessment model for cases of land disposal (e.g. landfill or land application). The HWIR would be applicable to Clean Salt products and could be used to effectively de-list these materials for land disposal.
Hanford tank waste is also subject to the requirements of the Atomic Energy Act of 1954, the Nuclear Waste Policy Act of 1982, and DOE Orders which are intended to assure compliance with these statutes. Although DOE and NRC regulations do not specifically address the recovery of useful materials from waste, DOE 5820.2A, "Radioactive Waste Management," states that waste containing amounts of radionuclides below regulatory concern (BRC) may be disposed of as non-radioactive. Previously DOE, EPA, and NRC collaborated to establish BRC or de-minimus levels but were unable to establish standardized release limits. Recently, DOE's Office of Environment, Safety, and Health issued guidance for determining release limits that meet requirements for radiation protection of the public and the environment (7).
PREFERRED DISPOSITION OF CLEAN SALT PRODUCT
Previous studies examined a wide range of alternatives for disposition of the clean salt product from use as fertilizer to direct disposal as waste (3-5). This study narrowed the list to technically feasible and practical alternatives that have clear regulatory dispositions. The preferred alternative for clean salt is recovery and reuse as a commercial chemical product.
Commercial sodium nitrate is supplied primarily from natural sources in Chile and is offered in two grades; industrial and agricultural. World consumption is about 300,000 metric tons/yr of industrial grade and 500,000 tons/yr of agricultural grade (8). Annual production of sodium nitrate from treatment of Hanford tank waste would be small by comparison at about 30,000 tons/yr.
Sodium nitrate is primarily used in agriculture as fertilizer to deliver nitrogen to the soil. It is particularly effective as a nitrogen source for cotton, tobacco, and vegetable crops The main industrial use of sodium nitrate is in the manufacture of explosives. It is used as an oxidizer in slurry-based blasting agents and explosives. Typical slurry explosives contain 10-15 weight percent sodium nitrate. Typical dynamite contains 44 weight percent. Sodium nitrate is also used in the manufacture of glass, fiber glass, enamels, and porcelain.
A key issue for the CSP is whether the product salts can be placed on the market for unrestricted commercial use. Radiation protection standards of DOE 5400.5 and the proposed 10 CFR 834, "Radiation Protection of the Public and the Environment," must be satisfied. DOE guidance requires that the effective dose equivalent resulting from the release of material not exceed 1 mrem/yr to any member of the public or a total of 10 person-rem/yr. As Low as Reasonably Achievable (ALARA) considerations must also be satisfied (7).
To assure the CSP meets requirements, it would be necessary to analyze a comprehensive set of possible end uses to determine whether dose rate criteria will be met. Clean salt product meeting specifications could then be released for those uses that are determined to be safe. The recommended approach is to analyze the primary industrial use of sodium nitrate, the production of explosives. Interestingly, an example of successful compliance with DOE guidance for release of materials is the recycle of explosives from DOE's Pantex Plant as discussed below.
Release criteria were developed for tritium contamination in high explosives generated as a result of weapons dismantling activities at Pantex. The release criteria state that explosive material can be released to the commercial market if the average bulk activity is less than 2300 pCi/g or removable surface contamination is less than 1,000 dpm/100 cm2. Depth profile experiments showed that when surface contamination was less than 1,000 dpm/100 cm2, the volume contamination was less than 2300 pCi/g.
To support development of release criteria for explosive materials, a pathway analysis for radiation dose from unrestricted release was conducted. Assuming the entire high explosive charge had bulk tritium activity of 2300 pCi/g, the maximum expected annual dose equivalent to any member of the public was less than 1 mrem and the anticipated dose workers was far below radiation protection requirements.
For the ALARA analysis, economic costs and radiological doses to members of the public were evaluated for viable disposition options. The analysis considered a detonation option and a recycling option. The economic costs strongly favored the recycling option. The individual and collective radiation doses were negligible for both options. Therefore, the Pantex Plant recommended that the explosive be released to the commercial sector for reuse.
OTHER DISPOSITION ALTERNATIVES
Based on this analysis, recovery and reuse of clean salt as a commercial chemical product is preferred because it is technically viable and achieves the greatest waste volume reduction and cost savings. Other alternatives are available but are less attractive from either cost or regulatory standpoints.
Conversion to Sodium Carbonate
This alternative was first proposed by Hendrickson (3) as a beneficial use of the clean salt recovered from Hanford tank waste. After separation and purification of sodium nitrate, the salt is converted to sodium carbonate. This is then used as an admixture in grout for structural backfill of the waste tanks after the waste has been removed. Several methods are possible for converting the nitrate to carbonate. Most are significantly less expensive than vitrification and disposal as LLW.
Total volume of Hanford tanks for backfill purposes is about 630,000 m3 (including dome space and double shell tank annuli) requiring about 1,260,000 metric tons of grout. The CSP would produce about 90,000 metric tons of sodium carbonate (7 wt% of total grout).
Carbonate conversion and grouting would be necessary for use as tank backfill. Use of sodium nitrate could cause nitrate contamination of soil and groundwater beneath the tanks. Grouting reduces the potential for the carbonate to leach out and increase migration of existing soil contamination. The conversion process also removes the hazardous waste characteristic of ignitability and may provide a basis for de-listing the waste.
According to Hanfords Tri Party Agreement, up to 1% the waste will be left in the tanks for closure (99% retrieval). Assuming volume and radioactivity are proportional, more than 1 million curies will be left in the tanks. Clean salt fill would add about 60 millicuries to the total. Given this situation, extensive decontamination of the salt may not be necessary to meet requirements of DOE 5820.2A, "Radioactive Waste Management". Fewer separation stages and relaxed product specifications would reduce cost of the CSP but these must be balanced with worker safety and ALARA considerations.
Conversion to Nitric Acid and Sodium Hydroxide
Clean sodium nitrate from the CSP can be processed electrochemically to produce two separate streams, nitric acid and sodium hydroxide. At Hanford, on-site demand for acid and caustic is about 20% of CSP production. This provides nitric acid for pretreating CSP feed and caustic for enhanced washing/leaching of Hanford HLW sludge.
Nitric acid and sodium hydroxide are bulk commodity chemicals with large quantities used annually. With the CSP, pure chemicals with extremely low levels of residual radioactivity could be produced. These materials will likely meet existing criteria for release of materials with radioactivity in volume, however this does not guarantee compliance with DOE guidance (7). To do so, end uses for these commodities must be analyzed to determine if they meet the 1 mrem/yr criterion and ALARA considerations.
Another option is to decontaminate less extensively and market the acid and caustic to other DOE facilities, NRC-licensed commercial facilities, or other qualified users throughout the world. Extent of radionuclide removal required would depend upon customer requirements and transportation regulations. Decontamination to < 2000 pCi/g would allow transport as non-radioactive material. Success of this option depends upon identifying a large set of end uses. For example, the modified CSP would produce enough nitric acid annually to process about 12,000 metric tons of irradiated nuclear fuel, substantially higher than the demand for that end use. Without other large volume uses, this option is not practical.
RECOMMENDATIONS FOR ACTION
The clean salt process is a viable option for treatment of tank wastes at Hanford and other DOE facilities based on laboratory testing performed to date. There is potential for substantial waste volume reduction and cost savings on the order of several billion dollars. This warrants further consideration and more detailed evaluation. Regulatory analysis indicates that clean salt recovered from the waste is exempt from hazardous waste regulations when useful products are produced. A mechanism and guidance are in place to determine whether the decontaminated clean salt product can be free-released for its primary commercial end-use, the manufacture of explosives.
Additional technical data are needed to consider integrating CSP into the TWRS technical baseline. A pilot scale process should be developed and operated using the same unit operations envisioned for the production process. This should be run first with simulated waste, then with actual waste. Typical decontamination factors and overall salt recovery factors need to be determined at the engineering scale. Chemical variations in the feed need to be evaluated and tested. More detail is needed in the CSP design concept to improve the cost estimate and sensitivity analyses should be performed to understand critical cost drivers.
Estimated dose and ALARA analyses are needed to develop de-minimus levels for residual radioactivity in the CSP product. For commercial re-use, levels will be based on the 1 mrem/year guidance provided by DOE. A similar analysis determined an acceptable release level for tritium contaminated explosives of 2300 pCi/g. Clean salt is capable of 2 pCi/g (total beta/gamma). If the CSP product used as backfill for tank closure, a higher level of radioactivity may be acceptable. The end state for tank closure must result in a dose of less than 25 mrem per year (DOE 5820.2A). For reuse in other nuclear processes, acceptable levels would be based on transportation standards and worker dose considerations.
Based on the laboratory data, the several engineering analyses performed to date, and this study, the Clean Salt Process shows great promise for application to Hanford Tank Waste. The process will substantially reduce LLW volume and would save billions of dollars in treatment and disposal costs. The preferred alternative for disposition of the CSP product is recovery of clean sodium nitrate for use in the manufacture of explosives.
The CSP also has potential for application to other DOE tank wastes since most have substantial soluble salt content. Extensive waste volume reduction and cost savings are possible. Reduced nitrate content would be advantageous for the residual LLW depending on the performance of the baseline waste form. The CSP also has potential for application to non-radioactive hazardous waste streams. This may represent a substantial opportunity for waste minimization and recovery of chemical products for beneficial re-use.
REFERENCES
ACKNOWLEDGMENTS
The authors wish to thank the US Department of Energy, Office of Science and Technology and Dr. Theresa Fryeberger for sponsoring this technology under the Efficient Separations and Processing program. We wish to acknowledge Dr. Dan Herting for his innovation and perseverance in developing the technology; Graham Maclean, Doug Hendrickson, Todd Lundsford and others for contributing to the development. The authors also wish to acknowledge DOEs Tanks Focus Area for supporting this study as part of Waste Policy Institute Contract No. DE-AC21-96MC33241. The clean salt process is applicable to tank wastes with high sodium nitrate content such as are found at Idaho, Oak Ridge, Hanford, and Savannah River Site.