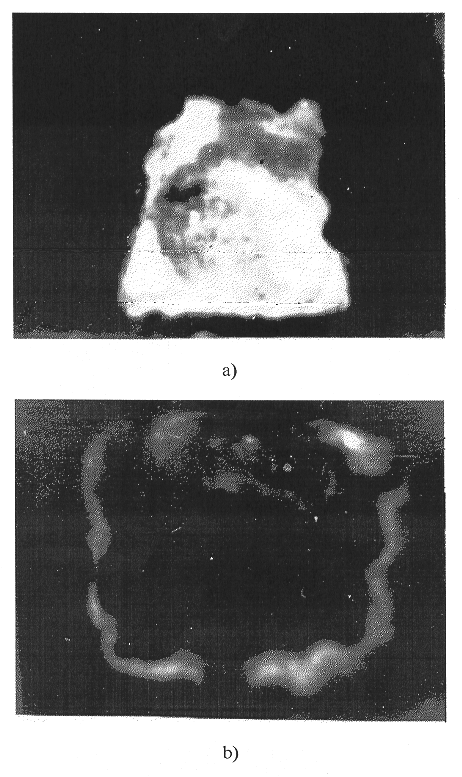
Fig.1. Autoradiographical images of
the metal surface before (a) and after (b) the thermosorption processing.
Igor A. Sobolev, Viktor M. Tivansky, Arcady G. Petrov, Olga
K. Karlina and Michael I.Ojovan
Scientific and Industrial Association "Radon",
The 7-th Rostovsky Lane, 2/14, Moscow, 119121, Russia,
Tel. (095)248 1680,
Fax (095)248 1941, E-mail oj@nporadon.msk.ru
ABSTRACT
Exothermic metallic compositions (EMC) were used in order to decontaminate radionuclide contaminated metal surfaces. Experiments were carried out with samples of carbon and stainless steel which were contaminated by 137Cs. Radionuclide distribution and aerosol releases were measured during the process of decontamination. High efficiency of the applied technique was found as well as absence of environmental contamination.
INTRODUCTION
The method of thermochemical decontamination of surfaces was first used for decontamination of radionuclide contaminated asphalt layers (1). In comparison with traditional methods of decontamination (2, 3), this technique is much easier for practical realization, is autonomous and ecologically safe. In this paper the method of thermochemical decontamination has been used for decontamination of radionuclide contaminated equipment.
The method of thermo-chemical desorption is realised by means of contact heating of the radionuclide contaminated surface of metal with the help of exothermic metallic compositions (EMC). The burning of these compositions on the surface of metal during 10 minutes results in a temperature of more than 600°C, thus causing radionuclide desorption from the surface of metal and diffusion into the EMC, with the following sorption in the colder layer of products of burning.
EMC are polydispersed powders of magnesium and its alloy with aluminum with the addition of nitrates of sodium or potassium and industrial oil. The detailed composition of EMC and their main physical and chemical characteristics can be found in (1,4).
THE MECHANISM OF THERMOCHEMICAL DECONTAMINATION
The analysis of micro thermocouple and thermogravimetrical measurements leads to the following presentation of the thermochemical decontamination of the surface of metal. The first stage is a comparatively quick (with the linear speed ~ 1 cm/s) spreading of the wave of burning from the initiation point all over the EMC surface due to its own oxidizer (the portion of which in the mixture is 2-3 %) and air oxygen. The first phase ends with the formation on the EMC surface of a sufficiently strong crust of residues consisting mainly of Mg and Al oxides and having a thickness of several millimetres. The second phase is a relatively slow (the linear speed ~ 1 mm/min) oxidizing of EMC mainly at the expense of air oxygen which diffuses from above through the layer of products of burning. The heat of the reaction of burning raises the temperature of the surface of metal to 600-800°C and more. At such temperatures, the radionuclide desorption from the surface of metal and transition to the gaseous phase occurs.
The distinctive feature of this process is the diffusion regime of EMC burning. By the time when the temperature of metal rises to 600°C, the air penetrability of the frame of the products of burning (crust of slag) decreases significantly and the diffusion flow of oxygen into the area of EMC burning is formed due to the peripheral inflow of air oxygen. This flow keeps the steam-like gaseous radionuclides under the layer of burning EMC. According to the assessments made, the linear speed of the peripheral flow exceeds values of 1 m/s, and the number of Peclet is very large Pe>>1, which means that the convection diffusion prevails over the Brownian movement. By the end of the burning process, this convection flow decreases, which is also accompanied by decreasing the temperature of the outer layer of products of burning compared with the central zone of EMC.

Fig.1. Autoradiographical images of
the metal surface before (a) and after (b) the thermosorption processing.
Owing to a high heat conductivity, the temperature of metal on the periphery of EMC is higher than the temperature of the slag frame. As a result, radionuclides diffuse in the gap between the slag frame and the metal from the hot central zone to the periphery, where they precipitate on the colder slag due to the forces of thermophoresis which act in the gap between the hotter metal and colder slag frame (see Fig. 1). With the temperature gradient ~ 100°C/mm, the effectiveness of thermoprecipitation, when the flow rate ~ 1 m/s, reaches 99%. For this reasons, it is expected that the carry-over of radionuclides to the environment will not exceed 1%.
EXPERIMENT
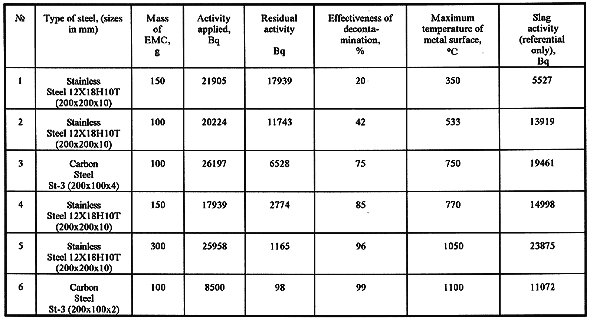
Experiments on thermosorption decontamination were carried out on samples of carbon and stainless steel of different thickness (with in 2 - 10 mm ) with a size of 100 x 200 mm. In order to simulate radionuclide contaminated a water solution of caesium nitrate (137Cs) was applied to the section of metal surface with an area of 100 cm2. The total radioactivity of the solution was 2x104 Bq (Table I). The metal was kept for 24 hours under normal conditions at a room temperature to complete drying.
Table I Data of Decontamination Process
To study the influence of the method of applying radionuclides and surface roughness on the effectiveness of thermochemical decontamination, carbon steel plates of sizes 100x70x4 mm underwent chemical etching. A paraffin frame at the sides of the samples kept the etching solution on the surface of metal. The etching was carried out during 15 days, the etching solution of NaNO3 (10 g/l) and NaOH (20 g/l) periodically added to the area inside the paraffin frame. As a result, a region of erosion and a crust-like layer of salts appeared on the surface of metal. After etching, the solution of 137Cs nitrate was applied to the surfaces of samples.
The decontamination of the samples was carried out in the following way. A layer of EMC of thickness 1 cm was placed on the surface of metal and then burnt in open air. The burning went on for 15-20 minutes, then the samples were cooled for another 20 minutes. The sintered layer which formed on the surface could be easily removed from the surface of metal by a putty knife.
The radiometric analysis of the slag and metal (before and after the
decontamination) was made with the use of a
 -spectrometer with a
multichannel analyzer AMA-03F.
-spectrometer with a
multichannel analyzer AMA-03F.
To study the distribution of radionuclides on the surface of metal, the autoradiography method was used. An X-ray film RETINA XBM of sizes 13x18 cm with emulsion 13 was taken as a photo detector. The film processing was carried out in a standard solution "Roentgen-2".
The registration of temperature fields on the surfaces being decontaminated was carried out with the use of chromel-alumina thermocouples, which junctions were welded into the metal to a depth of 1 mm. Electric signals from the thermocouples were detected by the 14-channel recording magnetometer H068 in the real time mode with the tape speed 4,75 mm/s. After the experiment, the information recorded by the recording magnetometer was loaded to the PC AT-386 memory with the use of a 12-digit intermediate device and with the tape speed 32 time as high as during the recording.
The process of thermochemical decontamination of metal surfaces was carried out in a cubic enclosure of volume 1 m3 equipped with an exhaust ventilation with an aerosol filter consisting of two layers of AFA-like Petryanov's cloth (the first layer was working, the second -controlling). After the decontamination, the mass increase of each filter owing to particulate material formed during EMC burning as well as their radiometry were determined.
RESULTS AND DISCUSSION
Results of a number of experiments on decontamination of metal surfaces are shown in Table I. The data show the applied and residual activity as well as the values of maximum temperatures of metal surfaces. The data on the slag activity should be considered as the reference ones, because the geometry of X-ray spectroscope specimens - metal plates and EMC slag - do not coincide.
The effectiveness of decontamination K is given by the formula:
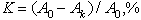 (1)
(1)
where Ao, Ak - are the activities of the metal surface in Bq before and after the decontamination, respectively.
Figure 1 shows autoradiographical images of the metal surface before and after the thermosorption processing. As a result, besides decreasing the radioactivity on the metal surface, the radioactivity is also redistributed from the central zone to the periphery, which is approximately the boundary of EMC. Such a distribution of radionuclides is in agreement with the above presented ideas of the mechanism of the thermosorption decontamination of metallic surfaces.
Figure 2 presents the time dependencies of the temperature of metal plates, mass rate of particulate release and the rate of EMC mass increase due to air oxygen (and, apparently, nitrogen - Table II). EMC mass was 50 g, the burning of which released
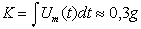 (2)
(2)
of particulate, being ~ 0,6 % of the initial mass. The increase of EMC mass takes place mainly during the phase of burning initiation when the wave of burning spreads over the specimen surface. During this period a good correlation between the rate of mass increase and that of particulate release is seen.
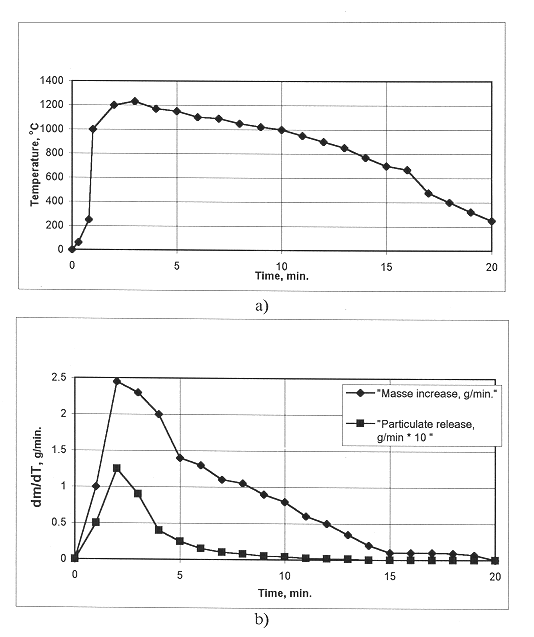
Fig. 2. Time dependence of
temperature of metal (h=2mm) surface (a) when using EMC (1 cm layer), rate of
EMC mass increase and mass rate of particulate release (b).
Figure 3 shows the time dependencies of the temperature of metal plates of sizes 200x200x10 mm. The junctions of thermocouples were placed in the geometric centre of plates at a distance of 1 mm from the upper surface. Temperature changes were made by changing the EMC mass burnt on the surface of metal.
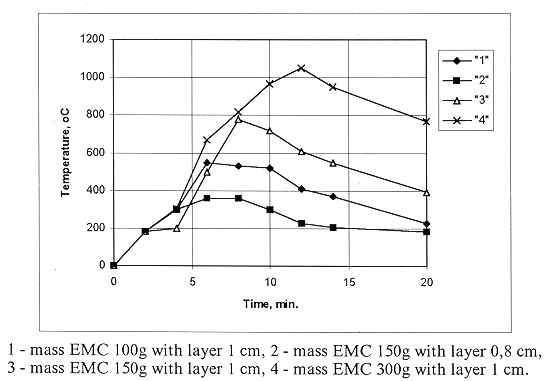
Fig.3. Time dependence of temperature
of heating at 12X18H10T steel plate by EMC; 1 - mass EMC 100g with layer 1 cm, 2
- mass EMC 150g with layer 0,8 cm, 3 - mass EMC 150g with layer 1 cm, 4 - mass
EMC 300g with layer 1 cm.
One should note that the heating of a plate of thickness 10 mm to a temperature of 1000°C requires no more than 5% of the EMC produced heat.
The data on the decontamination coefficient for alloy-free and stainless steel of thickness 2 mm, 4 mm and 10 mm are united into one function shown in Fig. 4.
Fig. 4. The dependence of
effectiveness of decontamination of alloy-free and stainless steel plates of
sizes 200x200 mm on the temperature of surface heating.
Thus, whatever the sort and thickness of steel specimens, the effectiveness of their thermosorption decontamination is definitely determined (for the given EMC) by the temperature of the surface.
In a number of experiments the influence of EMC slag adhesion on the effectiveness of decontamination was found. It was determined that doping the EMC with adhesion decreasing additives leads to decreasing the effectiveness of decontamination. The quantitative connection of these parameters will be found out in the future experiments.
For the specimens of carbon steel which underwent chemical etching in accordance with the above presented technique, the removal of transferable contamination was carried out with the use of cotton wool gauze plugs damped in distilled water. The specimens were wiped with the plugs for several times until the radiometer (UIM2-2 apparatus) with the detection unit BDB2-01 stopped indicating radioactivity. After measuring the residual radioactivity on the specimens, they were subjected to the thermosorption decontamination. The corresponding results for one of the specimens are shown in Table II.
Table II Results of Decontamination of an Etched Metal Specimen
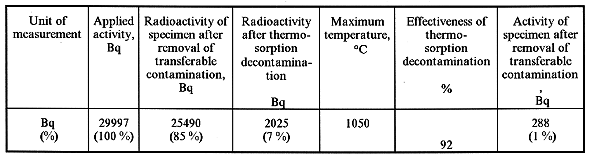
The analysis of the data shows that the etching results in the formation of unremovable contamination on the surface of a metal specimen. The contamination which can not be removed by a cotton wool gauze plug is decontaminated by the thermosorption technique with an effectiveness of ~ 92 % with the maximum temperature 1050°C, which is in a good agreement with the data shown in Fig. 4. Moreover, after the thermosorption treatment, the residual radioactivity can be reduced by an order of magnitude by wiping with a cotton wool gauze plug.
The analysis of the mass increase of analytical filters and their radiometry reveal small (1 % ) release both of radionuclides and mass during the thermosorption decontamination of metal surfaces contaminated with 137Cs.
CONCLUSIONS
Thus, the thermochemical technique, which uses EMC, ensures decontamination of 137Cs contaminated metal plates of thickness up to 10 mm with the effectiveness more than 90 %. During the process the majority of the radionuclides go from metal surface to a sufficiently strong but easily removable slag. There is no release of radionuclides with the particulate phase into the environment.
REFERENCES