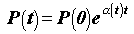 (1)
(1)
W. E. Kastenberg, P. F. Peterson, J. Ahn, J. Burch, G.
Casher, P. L. Chambré,
E. Greenspan, D. R. Olander and J. Vujic
Department of Nuclear Engineering
University of California, Berkeley, CA
94720-1730
B. Bessinger, N. G. W. Cook, F. M. Doyle and B. Hilbert
Department of Materials Science and Mineral Engineering
University of
California, Berkeley, CA 94720-1760
ABSTRACT
We systematically assess potential routes to autocatalytic criticality in geologic repositories. Our focus is on heterogeneous depositions of Pu and U away from emplacement having high concentration of fissile isotopes. If HEU or 239Pu are transported and deposited in concentrations similar to natural uranium ore, criticality can, in principle, occur. Removal of a small fraction of pore water provides a positive reactivity feedback mechanism which can initiate a super critical chain reaction. Rock heating and mixing of fissile material and rock can further increase reactivity significantly. However, at Yucca Mountain, it is highly unlikely that these configurations will occur; Pu transport would occur primarily as colloids and deposit over short distances. HEU solute can move large distances in the Yucca Mountain setting; its ability to precipitate into critical configurations is unlikely, due to a lack of active reducing agents. Uranium accumulation by ion-exchange with zeolite need be thoroughly considered. Appropriate engineering of the waste form and the repository can reduce criticality probability.
INTRODUCTION
Recently, the U.S. Department of Energy has initiated studies of the geologic disposal of 50,000 kg or more of separated excess weapons plutonium, that may be immobilized in glass or ceramic (1), and 210,000 kg, or more, of highly enriched uranium (HEU) from research and naval reactors (2). Original emplacements of fissile material in repositories will be made subcritical (keff < 1) by design by incorporating neutron absorbing materials and by controlling the quantity and geometry of TFM in each canister. C.D. Bowman and F. Venneri (B&V) postulated (3) scenarios for autocatalytic criticality caused by the reconfiguration, over millennia, of Thermally Fissile Materials (TFM) buried in the Yucca Mountain geologic repository. If the effective neutron multiplication factor keff of the system exceeds unity, the fission rate P will vary with time as,
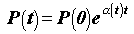 (1)
(1)
where the inverse period, or "time eigenvalue,"
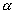 (t) is approximately equal
to
(t) is approximately equal
to 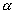 =
=
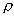 /
/![]() ;
;
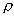 is the instantaneous
reactivity,
is the instantaneous
reactivity,
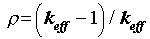 (2)
(2)
and
![]() is the effective neutron generation time. In autocatalytic chain reactions
is the effective neutron generation time. In autocatalytic chain reactions
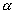 (t) increases, via positive
reactivity feedback mechanisms, as the fission density goes up. In all super
critical systems, the energy release terminates after negative reactivity
feedback mechanisms eventually force
(t) increases, via positive
reactivity feedback mechanisms, as the fission density goes up. In all super
critical systems, the energy release terminates after negative reactivity
feedback mechanisms eventually force
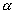 to become negative.
to become negative.
The critical configurations analyzed by B & V (3) consist of silica in which TFM is homogeneously and uniformly mixed. The silica, with or without some water, slows down the fast neutrons (primarily via elastic collisions with oxygen and, when present, hydrogen) to thermal or near thermal velocities, increasing the fission probability per nucleus of the fissile isotope. The B & V theory received intensive evaluation. These evaluations and earlier relevant studies are reviewed in Ref. 4.
We have performed an independent assessment of mechanisms and scenarios for underground criticality events (5-11). The study focuses on scenarios where TFM are transported away from the original emplacement locations and accumulated somewhere in the host rock. We address the following two questions, "Are there realizable configurations of TFM and moist rock in which autocatalytic criticality events could develop?" and, "In the Yucca Mountain geologic, hydrologic and geochemical setting, can these configurations occur?" An overall summary of our findings is given in Ref. 5. The present paper further elaborates on scenarios for the initiation of autocatalytic chain reactions, on the analysis of plutonium and HEU transport mechanisms and on possible engineering measures for further reducing the probability of formation of critical configurations.
AUTOCATALYTIC CRITICALITY EVENT TREE
Seven events would be required to cause autocatalytic criticality with rapid energy release, sufficient to vent radioactivity above ground. For a given scenario and waste form, if engineered or natural features prevent any of the seven events from occurring, venting of radioactivity becomes impossible.
First, the waste package must degrade before significant quantities of TFM undergo radioactive decay. For scenarios involving 239Pu the time scale for degradation is limited by the 24,400-year half life. For scenarios involving HEU spent fuel (and weapons-grade plutonium that has decayed to HEU), the main time constraint comes from regulatory requirements, because 235U has a 700-million-year half life.
Second, chemical processes must separate neutron absorbing poisons such as boron and 238U from the TFM.
Third, hydrologic processes must transport the TFM, either dispersing it into moderating material around the original waste emplacement, or carrying it away from multiple emplacements.
Fourth, a sufficient quantity of TFM must be available and transported.
Fifth, the TFM must be re-deposited in a critical configuration.
Sixth, upon reaching criticality, some mechanism must provide positive reactivity feedback as the system heats.
Seventh, the dynamic response of the system must keep the neutron multiplication factor keff above unity until sufficient energy has been released to be of concern. 10 CFR Part 60, which governs the deep geologic disposal of materials that may be capable of supporting criticality, requires the design to ensure that criticality is not possible unless at least two unlikely, independent changes have occurred. However, because most criticality scenarios would generate very small inventories of fission products compared to those originally placed in the repository, this requirement is over-conservative from a risk perspective and is under review (12). Because any significant risk from underground criticality would require a mechanism for dispersal to the atmosphere, we have assumed a criterion for unacceptable underground criticality events as the venting of radioactivity to the atmosphere.
For scenarios involving transport of TFM away from emplacements, the potential quantity of TFM is greater than the inventory in a single canister. We were unable to entirely eliminate two potential mechanisms that may deposit critical configurations of TFM from multiple emplacements. In this paper we address these two mechanisms: the potential for transport of HEU in solution in ground water, with precipitation in the far field; and the transport of plutonium-bearing colloids, with deposition away from multiple emplacements.
Only two of the potential waste forms can yield HEU: HEU spent fuel; and weapons-grade plutonium immobilized in glass or ceramic, after the 239Pu decays to 235U. Natural uranium ore deposits provide evidence for the configurations HEU might take, if it were to precipitate below a repository. For example, in the Peña Blanca uranium deposit in Mexico, pitchblende (UO2+x) is found in fault zones in fractured tuff as coatings, veinlets, stringers and disseminations. In deposits of this type, average uranium weight fractions can be 0.3 percent, reaching 10 percent locally (13). Figure 1 shows that these concentrations would be sufficient to reach criticality in infinite, homogeneous systems (kinf = 1) for uranium with enrichment greater than roughly 4%. For finite systems, neutron leakage somewhat increases the critical concentration of HEU.
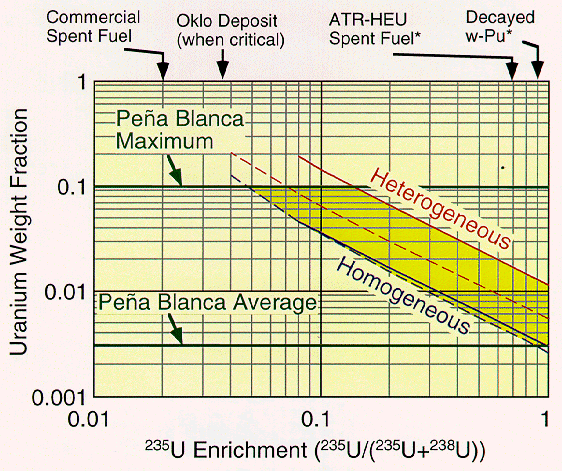
Fig. 1. Average uranium density
required to achieve criticality, kinf = 1, in tuff rock (2.2 g/cm3).
Blue and curves represent homogeneous and heterogeneous mixtures, respectively.
Solid and dashed stand for 0.2 and 0.1 g of water/cm3 of mixture.
Heterogeneous UO2+x coatings in parallel fractures spaced at 20 cm
require more plutonium. (*Approximate enrichment, diluted primarily with 236U.)
STATIC NEUTRONIC ANALYSIS FOR HETEROGENEOUS ACCUMULATIONS OF TFM
For the neutronics analysis presented here, geohydrologic transport processes were assumed to carry TFM from multiple canisters and deposit it in rock fractures as shown in Fig. 2 and as elaborated upon in the next section. Static calculations were performed for plutonium dioxide and uranium dioxide in Nevada tuff. TFM deposition are assumed to occur on surfaces of rock fractures, which are planar and parallel and equally spaced with each other. Water flows through fractures, and fills partially the void spaces of the rock matrix surrounding fractures. We have made a parametric study for the thickness of the TFM deposition layer, the fracture aperture, the fracture spacing, the TFM enrichment, and the water saturation in the rock matrix. Here we report on a limited subset of the cases considered, all pertaining to 239Pu. This almost 100% 239Pu is what will become of the weapons plutonium after few 6760 years half-lives of 240Pu. Information on effects of higher concentrations of 240Pu and on properties of HEU - tuff systems can be found, along with details of the assumptions made and results obtained in Refs. 5, 9 and 10. Specific goals of the neutronic parametric study included finding: a) the critical mass of heterogeneous depositions of TFM in tuff; b) the value of six reactivity-feedback mechanisms: water removal, TFM-temperature increase, rock-temperature increase, homogenization of TFM and rock, buildup of fission and transmutation products, and expansion; and c) identification of a scenario for an autocatalytic prompt super critical chain reaction.
Critical Mass and Volume
Table 1 gives the 239Pu mass and core volume of a number of configurations of 100% 239Pu and tuff. The cores of all these configurations is assumed to be spherical and to be surrounded by an infinite reflector of tuff. Each spherical core actually represents a large family of core geometries having the same neutron leakage probability. The TFM deposition is assumed to consist of PuO2 + Fe2O3 at 1:1 molecular ratio, 7.96 g/cm3 in density. The tuff density is 2.20 g/cm3. We selected the 2 m in radius spherical core having 254 kg 239Pu critical mass as the reference system for more detailed analysis. All the results that follow pertain to this system.
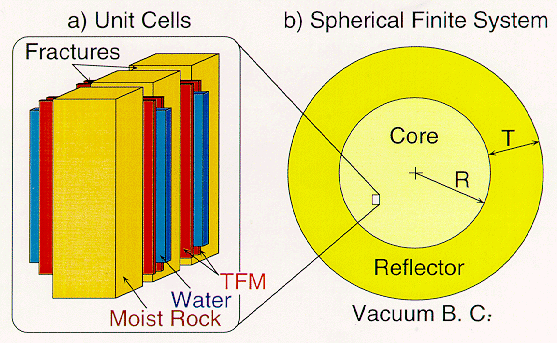
Fig. 2. Schematics of parallel
fracture lattice and the spherical finite system.
Table I. Selected Characteristics of Finite Systems
Reactivity Feedback Mechanisms
The change of keff and a due to a change in six system characteristics which may occur as either natural phenomenon or as a result of fission energy release was quantified. Following is a summary of our findings pertaining to keff:
Chain Reaction Initiating Scenario
The reference chain reaction initiating scenario considered is a natural drying-out process which takes place after a wet period. Neutrons from background radiation will establish a self-sustaining chain reaction. Eventually, the drying out process brings keff value to slightly above unity. As a result, the neutron population increases exponentially, although with a very long period. At this stage of evolution of the chain reaction (i.e., prior to the point in time in which the chain reaction affects the core temperature) there is no physical mechanism which can introduce negative reactivity (provided that no water is added to the core, that there is no drop in the ambient temperature, etc.). Ultimately, the fission energy generated from this slowly growing chain reaction starts increasing the core temperature. This temperature increase will drive a number of reactivity feedback mechanisms. In the reference system the three most effective reactivity feedback mechanisms are water removal, spectrum hardening due to rock temperature increase, and thermal expansion of the core volume. The first two mechanisms have a positive, while the core expansion has negative reactivity effect. The question is whether or not the reference system could evolve, under the combined effects of these three processes, to develop a prompt autocatalytic super critical chain reaction. Another question considered is what will be the system e-folding time at the time of initiation of pressure buildup.
Assuming that there is no interaction between the three reactivity feedback
mechanisms (i.e., neglecting non-linear effects), the gradient of an integral
system characteristic I (here representing either keff or
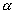 ), with respect to the
system temperature, T, can be expressed as
), with respect to the
system temperature, T, can be expressed as
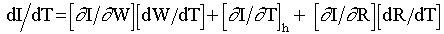 (3)
(3)
where W is the water concentration in the rock and R is the core radius. The
subscript h stands for spectrum hardening effect. The
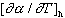 term was calculated from the
following expression:
term was calculated from the
following expression:
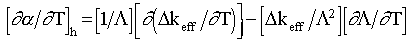 (4)
(4)
Using results from the preceding section and the linear coefficient of thermal expansion of water (1.85x10-4 [K-1]) and tuff (6.7x10-6 [K -1])11, we get for fully saturated rock,
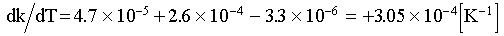 (5)
(5)
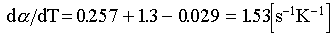 (6)
(6)
For unsaturated rock the first of the three terms in Eqs. (5) & (6) can
be neglected, giving dk/dT = +2.58 X 104 K1
and 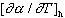 = 1.27 [s -1K
-1].
= 1.27 [s -1K
-1].
It is found that the overall initial temperature feedback effect will be
positive, with the dominant contribution coming from spectrum hardening. The
chain reaction could be self sustaining on prompt neutrons when keff
= 1 + 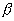 = 1.002. With a
dk/dT = 2.58x10-4[K-1] (See above), it will take a
temperature increase of less than 8°C to make the system prompt
supercritical. An additional heating of 1 K will bring the prompt e-folding time
to 1.26 -1 = 0.8 s.
= 1.002. With a
dk/dT = 2.58x10-4[K-1] (See above), it will take a
temperature increase of less than 8°C to make the system prompt
supercritical. An additional heating of 1 K will bring the prompt e-folding time
to 1.26 -1 = 0.8 s.
A second scenario postulates a "global" warming period which is
effecting the plutonium deposition site when its keff is unity or
very close to unity. The global warming can result from a climatic change. If
the overall temperature coefficient of reactivity near the ambient temperature
is positive (as it is for our reference system) this warming process could drive
the system to be delayed super-critical. For example, a mere 1 K increase in the
rock temperature will make the keff of an initially just critical
reference system to be 1.0003; the corresponding e-folding time of the neutron
population will be of the order of [0.0003]-1 S (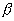 i /
i /
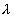 i) in which
i) in which
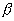 i and
i and
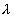 i are,
respectively, group I delayed neutrons yield and precursors decay constant. For
239Pu this period is of the order of 100 s. Thus, from this point on
it will not take a long time for the chain reaction to start heating the rock.
Further evolution of the system will proceed as described above for the first
scenario. In this scenario the rock can be saturated, whereas in the previous
scenario, the rock will be unsaturated at the initiation of the chain reaction.
i are,
respectively, group I delayed neutrons yield and precursors decay constant. For
239Pu this period is of the order of 100 s. Thus, from this point on
it will not take a long time for the chain reaction to start heating the rock.
Further evolution of the system will proceed as described above for the first
scenario. In this scenario the rock can be saturated, whereas in the previous
scenario, the rock will be unsaturated at the initiation of the chain reaction.
Summary
If large enough quantities of plutonium could be transported from disintegrated canisters and deposited "downstream" from the emplacement in concentrated enough form to make keff very close to unity, there are conceivable scenarios for natural phenomena to drive such systems to prompt, autocatalytic super criticality. A similar conclusion was arrived at for heterogeneous depositions of HEU in tuff. This latter finding was particularly surprising in view of the fact that HEU based systems considered so far all had negative spectrum hardening reactivity feedback.
RELEASE, TRANSPORT, AND DEPOSITION OF PU AND HEU
Three distinct aqueous processes would be involved in forming actinide deposits from repository emplacements: release from the waste form; transport in solution or suspension through the void volumes in the rock; and localized deposition from solution. These processes would have to be driven by water flowing through the repository from surface precipitation.
At Yucca Mountain, waste would likely be emplaced horizontally in mined drifts in massive metal canisters, located in unsaturated, highly-fractured welded tuff, a minimum of 200 m below the mountain surface and 300 m above the water table. The average vertical water infiltration rate is currently about 10-3 m3/yr-m2 (1 mm/yr), but is likely to rise by an order of magnitude during future pluvial periods (14). The present flow rate is equivalent to a few drops of water per hour per square meter of repository area. The flow distribution through the repository may be nonuniform as a result of the thermal perturbation from waste decay heat and fast-path flow through fractures. Regardless, on purely geometric grounds of this vertical influx would contact waste. The rest would either pass through the rock between drifts, fall in the air gap between waste and canister, or filter through canister debris. Engineered barriers such as graded backfill can provide additional protection against water ingress.
Based on typical fracture-augmented surface-to-mass ratios (0.01 - 0.02 m2/kg) and the rough mean of numerous estimates of the "long-term" glass alteration rate (~ 0.002 g/m2-d) (15), the lifetime of a glass log is ~105 years. The longevity of ceramic UO2 waste forms such as spent fuel, estimated by solubility-limited dissolution kinetics, is at least as long. As the glass or ceramic degrades, U and Pu would be released from the pristine waste form. Because of their low solubilities in water, they tend to remain on the waste surface as precipitates, most likely the dioxide for Pu, and for uranium, one or more of the numerous solid phases that U(VI) naturally forms.
Plutonium Behavior
The true solubility of PuO2 in water is almost certainly less than 10 ppb by weight (16), but because of the propensity of Pu to form colloidal particles, or, more likely, to be sorbed onto colloids formed from degradation of the waste form and canister, the apparent solubility can approach 1 ppm (19). These suspensions are not very stable, as evidenced by the following: I) when formed by degradation of Pu-containing glass, the solution concentration of Pu decreases with standing with a half-life of ~ 50 days (17); ii) the sorption coefficient of Pu on tuff of ~ 200 l/kg rock (2), which, though not giving concentrations sufficient for criticality, is still very large and probably reflects mainly removal of colloidal species; iii) almost all static or flow leach experiments involving Pu report the propensity of this element to adhere to experimental structures. In a repository setting, the most likely physical states of released Pu are: 1) mineralization on the altered glass surface; this form is easily spalled by water flow or even drying out (17); 2) particulates collecting on the drift bottom mixed with much larger amounts of rock rubble and iron oxides from the canister; and 3) in fractures within meters of emplacement.
Pu deposited in fractures can potentially form a critical configuration as shown by our static neutronic analysis. However, the expected short range of migration, because of colloid filtration and sorption on rock, means that such deposits would originate from one or at most a few emplacements. Because each emplacement would probably contain ~60 kg of 239Pu, much of which decays to soluble uranium before complete waste form degradation occurs, the probability of collecting the necessary critical mass from this limited source is very small. The small range of Pu migration is corroborated inferentially by the nearly complete immobilization of this element in the Oklo natural reactors since their activation two billion years ago (18).
The absence of long-range Pu migration is also supported by detailed diffusion-advection-sorption analysis of transport in a dual porosity system consisting of fractures and matrix porosity (8). Figure 3 illustrates the evolution of concentrations of 239Pu, 235U, and boron. In each rectangle, the fracture is located on the left vertical side. The right side represents the mid plane between two adjacent fractures. The vertical and dimensions are 200 m and 0.37 m, respectively. Water is assumed to flow in the fracture (the left side). Radionuiclides are transported by advection through the fracture, while diffusing into the rock matrix by molecular diffusion. Radionuclide movement my advection and molecular diffusion is retarded by sorption equilibrium between the solution phase and the solid phase.
Particulate Pu from many emplacements that has collected at the bottom of drifts may be washed by periodic high water flows to a low point in the repository where a critical mass could accumulate. However, the chief role of 239Pu in inducing criticality appears to be to produce the more mobile 235U by radioactive decay at the emplacement site.
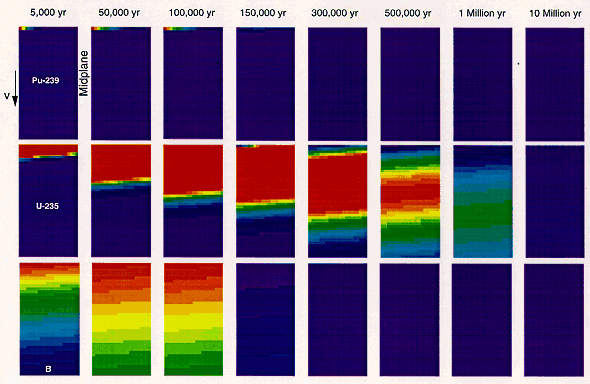
Fig.3. Migration of 239Pu, 235U, and
boron.
Uranium Behavior
The water chemistry at Yucca Mountain is conducive to high uranium
solubility: it is oxidizing, carbonated, and moderately basic. Solubilities
calculated from geochemical codes PHREEQE and EQ3/6 range from 10-9
to 10-4 M, depending on the solid phase assumed in the analysis
(19,20). Measured solubilities of UO2 or spent fuel in typical
oxidic, carbonated ground water center around 1 ppm by weight (Csat
~4x10-6 M) (20,21), too small for criticality. Colloid formation is
less important for uranium than for Pu. Assuming a water infiltration rate I =
10 mm/yr, and solubility-limited release of U from the waste form with a
dilution factor of f  0.1, the release rate of
U to the tuff underneath the repository is q = f I Csat
0.1, the release rate of
U to the tuff underneath the repository is q = f I Csat
 10-6 kgU/m2
-yr. The diffusion-advection- sorption analysis with this release rate provides
the spatial and temporal distribution of U in the rock, assuming that the
fracture surfaces are not sealed 8. The maximum concentration of sorbed U is
0.235(1-
10-6 kgU/m2
-yr. The diffusion-advection- sorption analysis with this release rate provides
the spatial and temporal distribution of U in the rock, assuming that the
fracture surfaces are not sealed 8. The maximum concentration of sorbed U is
0.235(1- )rKdCsat,
where
)rKdCsat,
where 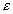
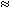 0.1 is the porosity and
0.1 is the porosity and
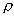 = 2200 kg/m3
is the density of the rock. With a U sorption coefficient Kd
= 2200 kg/m3
is the density of the rock. With a U sorption coefficient Kd
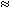 10 lit/kg3 , the sorbed
concentration is ~ 0.02 kgU/m3 or a weight fraction of ~ 10-5,
which is too small for a critical assembly, even for pure 235U.
10 lit/kg3 , the sorbed
concentration is ~ 0.02 kgU/m3 or a weight fraction of ~ 10-5,
which is too small for a critical assembly, even for pure 235U.
Some zeolite minerals are identified in Yucca mountain, and have the capability to sorb uranium to concentrations of around 0.7 wt% (22). From Fig. 1, it is found that, if uranium weight fraction of 0.7 % is achieved, around 50% 235U enrichment is sufficient for criticality. Further investigation would be necessary for criticality scenarios associated with zeolite minerals.
Whereas diffusion into the matrix would disperse uranium throughout the rock mass, sealing of fracture surfaces by mineral precipitates would permit these flow paths to deliver uranium to deep rock beneath the repository. Were such flow to encounter a zone of rock containing an accessible reducing agent (such as sulfides or other Fe(II) compounds), U(VI) compounds could be reduced to insoluble U(IV) and a pitchblende-like ore body formed. There is evidence that water in the aquifer of Yucca Mountain is, at least in some locations, chemically reducing (23). Furthermore, the tuff at all elevations contains small amounts of Fe(II), principally as magnetite (Fe3O4)24. The mountain-average concentration of this oxide is ~ 0.33 volume percent, which, if all used exclusively to reduce U(VI), could precipitate ~ 10 kgU/m3.
Despite these suggestions of the potential for uranium reduction, numerous factors indicate that such a process is highly unlikely. First, the usual and most geologically potent reducing agents, pyrite and organic matter, have not been detected at Yucca Mountain. Second, rock minerals containing reduced Fe in Yucca Mountain have survived ~ 2 million years of exposure to oxidizing water, suggesting that the Fe(II) is not accessible. Third, although some of the reducing components in the rock could be exposed by thermomechanical perturbations due to the repository or because of seismic activity, it is very unlikely that the entire charge of newly-accessible Fe(II) could be utilized to reduce U(VI). The concentration of dissolved O2 in Yucca Mountain water above the aquifer is ~ 100 times larger than that of uranium, although kinetic factors may favor uranium reduction over O2 reduction. However, radiolysis of the descending ground water and air by alpha decay of 239Pu produces very potent oxidizing agents (e.g., H2O2 and NO3- ) at concentrations comparable to those of uranium. In contrast with the roll-front mechanism responsible for many ancient uranium deposits, Yucca Mountain does not appear to contain sufficient quantities of reducing rock or reduced pore water to overwhelm the strong oxidizing agents expected in the water from the repository, thereby permitting uranium precipitation.
If, despite the above contraindications, a volume of reducing rock in the
path of uranium-laden ground water from the repository becomes chemically
activated, the time to accumulate a critical mass is Mcrit t/qA,
where q is the uranium release rate from the repository and A is the
projected area of the reducing zone. Taking Mcrit
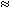 100 kg 235U, A
100 kg 235U, A
 10 m2 and q = 10-6
kgU/m2-yr, the time to achieve static criticality is ~ 107
years. This estimate does not account for "focusing" of the descending
water which could occur because the relatively impermeable basal vitrophyre
layer below the repository is penetrated only by a small number of fractures.
Focusing of the flow reduces the time required to achieve a critical mass, but
also reduces the probability that the flow would encounter a zone of active,
accessible, reducing rock.
10 m2 and q = 10-6
kgU/m2-yr, the time to achieve static criticality is ~ 107
years. This estimate does not account for "focusing" of the descending
water which could occur because the relatively impermeable basal vitrophyre
layer below the repository is penetrated only by a small number of fractures.
Focusing of the flow reduces the time required to achieve a critical mass, but
also reduces the probability that the flow would encounter a zone of active,
accessible, reducing rock.
Summary
The probability of accumulation of a critical mass of Pu or HEU in the Yucca Mountain tuff by the action of geologic processes on glass or ceramic UO2 waste forms is likely very small. Pu is expected to remain close to the emplacement site and to decay to HEU before the waste form matrix is completely degraded. Uranium will eventually be removed from the repository by water, but will be dispersed into the rock by diffusion and sorption at concentrations well below that required for criticality, except potentially in zeolite minerals. The likelihood and implications of uranium accumulation by ion exchange with zeolite need be thoroughly evaluated.
ENGINEERING STRATEGIES
Though the probability of forming critical deposits of TFM at Yucca Mountain appears exceedingly low, engineered systems can further reduce the probability, and may also provide a simpler basis for meeting licensing criteria.
Criticality at Original Waste Emplacement - Insoluble Poisons.
An engineered strategy for this case is to provide neutron absorbers with lower or comparable solubilities with respect to plutonium and HEU in the waste form.
HEU Precipitation in Far Field - Dilution.
Dilution of 239Pu or HEU with depleted uranium (DU) is an effective method of preventing criticality. Over 80% of the uranium ever mined exists as the tails of uranium enrichment plants, and is readily available as UF6. This material has no current large-scale use and is essentially free of charge.
To be effective in protecting Pu wastes from criticality, sufficient DU must remain at the emplacement site during the lifetime of 239Pu. In the current design of the Yucca Mountain repository, the air gap between the canister and rock is large enough to accommodate 50MT of DU per meter of tunnel length. With the infiltration rate and uranium solubility used above, the lifetime of this quantity of uranium dioxide is approximately 10 (9) years. Thus a much smaller quantity of DU would satisfy the 239Pu lifetime requirement of 10 (5) years. The potential of separation of DU from fissile material does not arise with HEU wastes, because both are chemically identical (even metallic HEU spent fuel will first be converted to the stable oxide upon contact with water).
At Yucca Mountain 238UO2 pellets could serve as the gravel in a graded backfill system, discussed below. For other repositories, alternative strategies could be adopted, such as integrating DU directly in glass waste forms.
Pu Colloid Transport Away From Emplacements - Immobilization.
Engineered backfill systems can potentially immobilize Pu colloids and allow their decay. Isolated failure of a backfill system could be acceptable, because the Pu release would be limited to a single emplacement.
Capillary barriers formed by the interface between sandy silt and gravel are now under study for use in a graded-backfill system for Yucca Mountain (25,26). A gravel backfill would be placed below and over horizontal MPC canisters prior to repository closure, after a several-decade retrievability period. Silty sand would be emplaced over the mounded gravel. Recent experiments have shown that recharge water flows up to 6.0 ml/hr/cm2, or fast path fracture flows up to 15 liters/hr, can be directed into the sand, and the water wicks laterally through the sand without causing saturation. This prevents water penetration across the capillary interface into the gravel (27).
By eliminating advection of water to the waste form, this system prevents mobilization of colloids. The performance of such systems has been demonstrated both by human analog structures--burial mounds over 1300 years old (28)--and by natural graded-fill analogs--protected pockets in gravel layers under finer-textured soil horizons that have resisted the penetration of liquid-water flow for tens of thousands, and in some cases, hundreds of thousands of years.
Use Pu to Fuel Power Reactors.
Use of the weapons Pu to fuel commercial power reactors and recycling of the fissile material from spent fuel provides an effective engineered solution; it may significantly reduce the probability of underground criticality accidents.
CONCLUSIONS
We can conceive of a large number of geometric and material configurations of TFM with tuff rock and water which are critical and in which a prompt super critical autocatalytic chain reaction could be established. However, at Yucca Mountain it is highly unlikely that Pu will travel large distances as a solute to form these configurations; Pu is more likely to be transported as colloids, and be redeposited close to its original emplacement. Additionally, 239Pu will decay to 235U before most of the glass waste form is leached. It is expected that commercial spent fuel behavior is similar.
Water chemistry at Yucca Mountain is conducive to high uranium solubility, hence 235U can move large distances as a solute. However, at Yucca Mountain, once uranium is in solution it is unlikely to precipitate into these critical configurations due to lack of reducing agents. Nevertheless, accumulation of critical mass of uranium by ion exchange with zeolite can not be ruled out without further study.
Engineering of the waste forms and the repository can further reduce the likelihood of the formation of critical emplacements with positive feedback. Important topics remain to be investigated theoretically, including the potential for TFM deposition in repository back-fill material, the minimum size for critical systems with positive reactivity feedback; concentrations of different poisons and DU which will minimize probability of formation of critical configurations; and dynamic response and energy release, particularly for small TFM deposits. The composition of Pu pseudo colloids, their mobility in rock fractures, the efficiency of their removal from infiltrating water are topics that must be explored by experiment.
ACKNOWLEDGMENTS
This work was performed under the primary support of LANL UCDRD funds. The Campus Laboratory Collaboration Program of the University of California, also supported part of this work. We gratefully acknowledge the researchers who provided useful information and input, including C.D. Bowman, D.K. Parsons, W.R. Stratton, and F. Venneri of LANL; T.S. Carman, W. Glassley, and R. Van Konynenburg at LLNL; R.P. Rechard at SNL; J. Apps at LBNL, F.F. Peterson at UNR, and J.L. Conca at WSU.
REFERENCES