Table I. Diffusivities (cm2/s) for Nitrate Containing
Polyethylene 
Phillip I. Pohl, Wu-Ching Cheng, Tim Wheeler, Robert D.
Waters
Sandia National Laboratories;
ABSTRACT
A scoping level evaluation of polyethylene encapsulation and vitreous waste forms for safe storage of mixed low-level waste was performed. Maximum permissible radionuclide concentrations were estimated for 15 indicator radionuclides disposed of at the Hanford and Savannah River sites with respect to protection of the groundwater and inadvertent intruder pathways. Nominal performance improvements of polyethylene and glass waste forms relative to grout are reported. These improvements in maximum permissible radionuclide concentrations depend strongly on the radionuclide of concern and pathway. Recommendations for future research include improving the current understanding of the performance of polymer waste forms, particularly macroencapsulation. To provide context to these estimates, the concentrations of radionuclides in treated DOE waste should be compared with the results of this study to determine required performance.
INTRODUCTION
The objective of this work is to identify potential performance benefits for disposal of polyethylene and vitrified (i.e., glass) mixed low-level waste forms relative to a grout waste form for water and intruder pathways. Estimated performance of polyethylene and glass are determined and compared with previously determined grout performance estimates. Estimated performance and performance comparisons are based on typical radionuclides found in mixed low-level waste that may be important for the water and intruder pathways.
The Performance Evaluation Methodology (PEM) used to evaluate grout in the mixed low-level disposal project (1) is applied in this work. Through a simplified reverse calculation, PEM started with the 4 mrem/yr drinking water limit at the 100 m performance boundary. This limit was converted to a radionuclide-specific maximum permissible concentration in groundwater at the performance boundary. Radioactive decay, and water and source concentration reduction factors were applied to determine the maximum permissible radionuclide concentration in the disposed waste form. PEM assumed a generic, shallow trench with waste stacked 5m high in the trench. In addition to the groundwater protection standard, the limit of 100 mrem/yr to an inadvertent intruder was also treated in this task. The intruder scenarios used in the present work are the post-drilling scenario for glass and polyethylene and a new "necklace" scenario for 2 cm glass beads.
The two sites selected for the performance estimates, Savannah River and Hanford, have significantly different soil and hydrogeological characteristics. Radionuclides selected for use in this task are H-3, C-14, Cl-36, K-40, Se-79, Sr-90, Tc-99, I-129, Cs-137, Pb-210, U-238, Np-237, Pu-239, Pu-241, Am-241. In this work, the volumetric basis of the contaminant loading definition is used with the activities of the specific radionuclides representing their mass. A five-part approach was used in this work (2): 1) Identified the dominant radionuclide release mechanism and estimated radionuclide leach rates; 2) Developed a radionuclide release model that provided a source concentration reduction factor; 3) Developed inadvertent intruder scenarios; 4) Determined maximum permissible radionuclide concentrations; and 5) Compared maximum permissible radionuclide concentrations in polyethylene and vitrified glass waste forms with those previously determined for the grout waste form (1).
RADIONUCLIDE RELEASE MECHANISM AND RATES
In general, a waste form is composed of the stabilizing matrix (e.g., polyethylene) and the waste. The waste is composed of the radioactive contaminants and the remaining waste constituents. The definition for waste loading used in this work is the amount of waste residue divided by the amount of waste form. Contaminant loading is defined in this work as the amount of radionuclides divided by the total amount of waste form.
Radionuclide Release Mechanisms
Polyethylene is an inert thermoplastic material used for encapsulating wastes for safe long term storage (3). It has a melting point of 120°C and processing temperature range of 120 - 150°C (4). As such, it is not susceptible to chemical interactions between the waste and binder and results in a monolithic solid waste form if compatible with the waste. Wastes can be microencapsulated or macroencapsulated in polyethylene.
There is little information available about radionuclide release mechanisms for macroencapsulated polyethylene. Macroencapsulated waste is used as a waste form for disposal into the sole commercially-permitted mixed waste disposal facility (Envirocare, Inc, in Utah). At this facility, regulations related to waste form performance are those of the Resource Conservation and Recover Act (Toxicity Characteristic Leach Procedure or TCLP); hence, investigation of release mechanisms is not a consideration since the waste form passes the TCLP test (5). Contaminant permeation through a continuous polyethylene barrier would probably be the release mechanism. There is a low probability that this contaminant permeation would result in a large rate since polyethylene absorbs almost no water under experimental conditions (6). Based on this conceptual model, a recommendation to Envirocare, Inc. was to use 1 inch of polyethylene around the perimeter of the encapsulated waste (7). Envirocare's decision was to use 2 inches instead as a waste form requirement (5).
The dominant release mechanism for polyethylene is leaching by diffusion. Performance data for polyethylene to date has been based primarily on the Accelerated Leach Test (ALT) (8). Results of the ALT are typically plotted as the cumulative fraction of contaminant leached versus time. Treybal (9) gives a textbook method for determining cumulative fraction leached as a function of a composite parameter consisting of the diffusion coefficient, the leaching time and a waste form dimension. This mechanism is a strong function of size, releasing at a higher rate as the waste form dimensions decrease. Estimates of the time scale for which polyethylene is considered effective for encapsulation without external forces are on the order of 1000 years (10). With time, crosslinking of the long polymers takes place and embrittlement in the polyethylene ensues, leading to the possibility of cracks in the polyethylene. Crack formation is enhanced by physical and radiation stresses. Physical stresses are assumed to be minimal. Degradation of physical properties of polyethylene by gamma irradiation and ultraviolet light, in combination with oxidation, has been observed (11). UV light should not be a factor for disposed polyethylene waste forms. However, radiation could be significant for highly radioactive waste disposal, since the Nuclear Regulatory Commission (NRC) Class C limits on activity for low-level wastes (10 CFR Part 61) are presently about two orders of magnitude greater than the dose rates predicted to create radiation damage in polyethylene wire coatings (12).
The dominant radionuclide release mechanism for vitrified waste is dissolution of the glass (13). Extensive dissolution data are available as a function of composition, temperature and solution conditions (pH, etc.). As the glass matrix dissolves, the bound radionuclides are released along with silicic acid (H4SiO4) into the solution. This phenomenon is directly proportional to the water accessible surface area of the waste (14). Upon cooling the vitrified waste, cracks can develop in the glass (15). Cracking of glass is a well recognized phenomenon but has yet to be completely quantified as a function of cooling rate. Increasing the surface area by a factor of five (16) and 20 (17) have been suggested. In the model below, we use a factor of 20 for the 60 cm canister glass form and no increase for the 1 cm pellets. These are equivalent to cooling for 40 hours and 1 hour, respectively (18). The effect of glass composition on release mechanisms has also been modeled (19,20). In general, the more silicon in the glass, the more durable the waste form.
Radionuclide Release Data
Data using the ALT have been gathered (8) for sodium wastes microencapsulated with polyethylene. The cumulative fractions of sodium leached are 0.15, 0.32 and 0.52, respectively, using 50, 60, and 70% loading. Table I gives diffusivities found (4) for nitrate containing waste. The data indicates diffusion increases with waste loading and temperature.
Table I. Diffusivities (cm2/s) for Nitrate Containing
Polyethylene 
All radionuclides are given the same diffusivity for two simple reasons. First, there is little experimental data which compares the leaching of different contaminants from polyethylene. Hence, the majority of the parameters would be speculative. Second, and maybe more importantly, the contaminants must be solubilized before diffusing out of the waste form. In this situation, the contaminants are molecular sized while the dimensions of the pores in the waste form are much larger (on the order of a micrometer). So, in reality, polyethylene probably imparts no discriminating factor on different radionuclides.
The forward rate of leaching for a vitreous waste form is determined similarly to the ALT (21). That is, the water used in solution is exchanged frequently, thus producing the greatest possible leaching conditions. On the other hand, the solubility limit of a particular radionuclide can limit this release, termed by some as the retention factor (17). A cumulative fraction of calcium leached from glass of 6.2(breve)10-7 using the ALT which equates to a forward rate of 4(breve)10-4 g/m2-d has been recorded (8). Mazer and Walther computed the linear dissolution rate for pure silica glass to be 7.6(breve)10-6 g/m2-d at a pH of 4 and temperature of 40°C (6(breve)10-7 g/m2-day corrected to 20°C) (22). The data showed that this value was approximately constant to pH 7 and should represent a lower bound on this parameter since pure silica is the most durable of the borosilicate glasses. Release rates for glass used in storing Hanford's low activity waste have also been defined (23). For the glass LD6-5412, the forward leach rate is 1.0(breve)10-4 g/m2-d at 20°C and pH 7, with pH and temperature dependence described by exponential and Arrhenius functions, respectively. A value of 2.5(breve)10-3 g/m2-d was assumed for evaluations, which consider the forward rate and a saturation condition at 90°C (3(breve)10-6 g/m2-d at 20°C assuming 20 kcal/mol activation energy) (17). Based on this discussion, we assume a conservative estimate for the long-term corrosion rate of glass is 1.0(breve)10-4 g/m2-d.
Review of Inadvertent Intruder Analyses for Shallow Land Burial
For low-level and mixed waste disposal, the most prevalent location for disposal is the shallow subsurface. In this section, a list of recent performance assessments (PAs) for low-level or mixed low-level waste is given. Doses for intruders are generally by ingestion, inhalation, and external exposure. In the Oak Ridge PA four scenarios were considered: the resident, agriculture, the post-drilling, and the discovery scenarios (24). At Savannah River, (25), three chronic exposure scenarios (agriculture, resident, and post-drilling) were included in the PA, giving similar results to (24). Post-drilling and post-excavation scenarios were evaluated at Hanford (26). The governing equations for these doses are linear combinations of dose conversion factors so that each transfer mechanism can be easily assessed. In the draft Hanford glass performance assessment, the parameters and scenario assumptions are given and explained (27). The scenarios are post-drilling and resident as in (26). The difference in treating the glass waste form over the cement of (26) is that some of the pathways are neglected. Since the glass will not be as finely ground as cement, then the ingestion and inhalation pathways are assumed neglible. Hence, glass will do better than grout with respect to intrusion. These results were reflected in the waste acceptance limits (28). In (1), homesteader and post-drilling are used and the waste form is a grouted solid. A basic assumption of each scenario is that the intruder cannot distinguish between the waste and the native soil. Assumptions similar to those above regarding exposure are made.
Since there is consideration to make glass waste forms consisting of 2 cm beads, a necklace scenario was developed for this work. Under this scenario, some future population finds the containers of glass beads, does not realize the danger involved and constructs a necklace from them. Since this is a scoping level evaluation, complete details of all possible mechanisms were not included, but rather a simple and conservative evaluation made.
SOURCE TERM MODELS FOR MAXIMUM RELEASE RATE
The Performance Evaluation Methodology (PEM) source term model provides the correlation between radionuclide concentrations in the waste form and concentrations in the leachate that exits the bottom of the disposal facility. The source model is used to formulate the Source Concentration Reduction Factor, CRFSource:
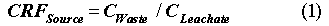
where CWaste is the concentration in the waste form for each radionuclide averaged over the entire volume of waste in the disposal facility (µCi/L), and CLeachate is the corresponding concentration in the leachate for each radionuclide as it exits the bottom of the disposal facility (µCi/L).
Performance Evaluation Methodology General Grout Model
For grout, an accepted correlation between waste form and leachate radionuclide concentrations has been established (1):
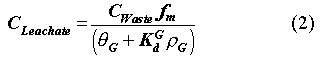
where 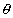 G =
0.3 is the volumetric water content of the grouted waste form (mL/mL); KGd
is the radionuclide-specific distribution coefficient (i.e., solid/liquid
partition coefficient) in the grout (mL/g);
G =
0.3 is the volumetric water content of the grouted waste form (mL/mL); KGd
is the radionuclide-specific distribution coefficient (i.e., solid/liquid
partition coefficient) in the grout (mL/g);
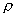 G = 1.8 g/cm3
is the dry bulk density of the grouted waste form; and fm = 2/3 is
the mixing fraction, defined as the ratio of the volume of waste disposed in a
unit volume of the facility trench. The source concentration reduction factor,
CRFSource, becomes:
G = 1.8 g/cm3
is the dry bulk density of the grouted waste form; and fm = 2/3 is
the mixing fraction, defined as the ratio of the volume of waste disposed in a
unit volume of the facility trench. The source concentration reduction factor,
CRFSource, becomes:
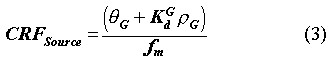
Encapsulated Polyethylene Waste Form Source Term
The model chosen for microencapsulated polyethylene is a function of waste loading, and waste form size. To tie into the performance evaluations, it is most useful to have the dependent variable in terms of leachant concentrations rather than fraction leached. In order to accomplish this translation, a mass balance was used, stating that what leaves the waste form goes into the infiltrating water:
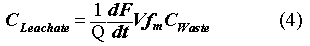
where Q is the flow rate of water through the waste site (m3/yr); F is the fraction of contaminant leached; t is time (yr); V is the volume of the waste form (m3); and fm is the same facility waste fraction from above. The PEM arrives at concentrations by assuming that the contaminants in the waste forms are not depleted with time, so that the source term is constant. Hence, from Eqs. 1 and 4, the concentration reduction factor will be:
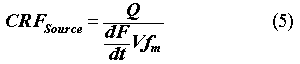
where dF/dt is assumed to be constant thoughout the period of performance.
Vitreous Source Term
The glass leach model assumes that radionuclides can only be released from properly formulated waste glass as a result of breakdown of the glass network. The fraction (F) of a canistered waste glass that corrodes per year after exposure to repository groundwater is (17):
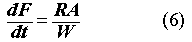
where R is the glass corrosion rate (g/m2-yr); W is the mass (g) of the glass in a canister; and A is the surface area (m2) of the glass contacted by water. A/W can be replaced by the specific surface area Asp which is a function of the degree of cracking discussed above. Hence, the CRFSource, is computed with the same formula as for the polyethylene case:
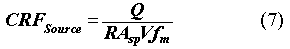
Since the performance evaluation is meant to provide conservative analysis, the forward dissolution rate is used as the release rate. Any effects of crystallization and solution pH on the glass release rate are neglected. The forward rate for a borosilicate glass waste form is assumed to be 0.0001 g/m2/d at the baseline loading of 30 wt%. This value is divided by 10 for low loading (10%), based on the assumption that a more durable glass could be formulated. It is multiplied by a factor of 10 for higher loading (50%) since the composition will likely cause a less durable glass to form.
INTRUDER SCENARIOS
The first scenario is the post-drilling scenario reviewed above in that a person drills a well through the waste site and distributes the residual soil over a given area of land. Since the drill hole has contacted the waste, the soil is contaminated with an equivalent concentration of radiation as the waste. Upon spreading, the soil is mixed with the topsoil. From here, radionuclides are released from their glass matrix slowly. The fractional release rate (0.001/year) was based on a forward leach rate of 0.0001 g/m2/day, and a cube of size 0.1mm. Inhalation mechanisms were neglected. For the polyethylene waste form, the release is immediate upon drilling; so, the results are the same here as for the grout post-drilling case. The parameters used are those given in (27).
The generic intruder equation for the permissible concentration in the waste (Cw) based on an intrusion scenario for a specific radionuclide is as follows:
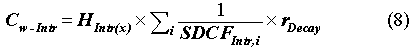
where Cw-Intr is the concentration of radionuclide in the waste disposal unit(mCi/m3); HIntr(x) is the total intruder annual dose for scenario x(rem/yr); SDCFIntr,I is the radionuclide-specific scenario dose conversion factor; and rDecay is the term accounting for radioactive decay prior to intrusion at 300 years. These intruder doses are calculated as in Appendix D of (29) and are not reproduced here. The only difference is in which doses are employed under the alternative waste-form scenario.
Since glass waste forms consisting of 2-cm beads are possible, a necklace scenario was developed. In this case, a string of radioactive beads are worn around the neck of the intruder for 8 hours one day per week. The dose received is the sum of all penetrating radioactivity over this time. For this scenario, the total intruder doses occur from direct external exposure. The dose of interest is the total effective committed dose (TECD, (30)) to the thyroid. The direct external dose was calculated by modeling each bead as a point source from which gamma energy was directed in all directions. Decay daughter radiation was factored into the dose calculations. To predict the incident radiation, the thyroid was modeled as a 4-cm by 8-cm rectangle and at a distance of 8.2 cm from each bead due to the diameter of the necklace (31). No energy attenuation in the waste medium of the beads nor in the tissue between the beads and the thyroid was assumed. The scenario dose conversion factor (SDCF) can be modeled as the specific gamma-ray constant. Beta radiation only contributed significantly to the Sr-90 dose. The equation for the permissible concentration in the waste (Cw) for a specified radionuclide is as follows:
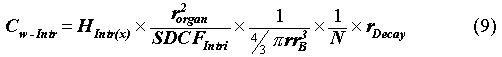
where SDCFIntr,I is the radionuclide-specific scenario
dose conversion factor, in this case the specific gamma-ray constant,![]() ,
rem-m2/µCi-yr; rorgan is the
distance to the thyroid gland; rB is the radius of the
necklace beads; and N is the number of beads in the necklace. To
calculate the Cw-Intr in Eqs. 8 and 9, the annual dose Hintr(x)
is set equal to the performance measure (i.e., 0.1 rem per year chronic dose).
,
rem-m2/µCi-yr; rorgan is the
distance to the thyroid gland; rB is the radius of the
necklace beads; and N is the number of beads in the necklace. To
calculate the Cw-Intr in Eqs. 8 and 9, the annual dose Hintr(x)
is set equal to the performance measure (i.e., 0.1 rem per year chronic dose).
COMPARISONS OF WASTE FORM PERFORMANCE
The concentration reduction factor for the groundwater pathway (CRFw) are 45 at Hanford and 5.1 at the Savannah River Site. With this factor, the maximum permissible concentrations in the leachate from a generic trench is established. The travel times are used to compute the decay of the radionuclides and have been obtained from (29). The pathway dose conversion factors (PDCF) are used to convert concentrations (mCi/m3) to dose (mrem/yr) assuming a 2-liter per day drinking rate and also taken from (29).
Source Concentration Reduction Factors for Encapsulated Polyethylene
Based on the diffusivities in Table I, results of the polyethylene microencapsulation performance in terms of the source concentration reduction factors at 20°C are given in Table III. There are two geometries evaluated, the 2 m diameter by 2 m high cylinder (4) and a 1x1 m cylinder, which is more likely to be used since it is roughly the size of a 55 gallon drum.
Table II. Polyethylene Source Concentration Reduction Factors (CRFSource)

It should be noted that the diffusivity used for the 30% loading was extrapolated from data at 50, 60, and 70% and ANS 16.1 leach index. Doubling the dimensions of the polyethylene waste form increases the CRFSource by 4-5 times. This increase is characteristic of the solute diffusion mechanism.
Source Concentration Reduction Factors for Vitrified Waste
Using the forward leach rates at 20°C identified above, the source concentration reduction factors in the two waste form configurations are given in Table III. Assumptions include a 40-hour controlled cooling time for the 60-cm canistered waste, which gives rise to a relative surface area increase of 20. The 60-cm canister has a higher CRFSource than the pellet form because of its lower specific surface area, even though it was assumed that the beads suffered no cracking.
Table III. Glass Source Concentration Reduction Factors (CRFSource)

Summary of Water Pathway Analysis
The permissible concentration in the waste form was computed as
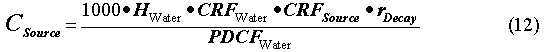
where the CRFWater and PDCFWater are described above. The rDecay is computed at the travel times. The regulatory limit HWater is 0.004 rem/yr. The resulting permissible concentrations of radionuclides in waste are shown in Table IV for polyethylene at 50 wt% WL (1 x 1-m cylinder), glass at 30 wt% WL (60 cm cylinder), and grout at 50 wt% WL.
Table IV. Permissible Waste Concentrations (µCi/m3)
in Polyethylene, Glass and Grout

The relative performance of polyethylene to grout depends on the specific radionuclide evaluated, as shown in Table IV. For Cl-36 and K-40, polyethylene performs approximately 2 orders of magnitude better than grout. For U-238 and Np-237, grout performs better than polyethylene. The relative performance of glass is also dependent on the selected radionuclide. For K-40, Se-79, and Tc-99, glass performs 3 to 4 orders of magnitude better than grout. For U-238, Np-237, and Pu-239, glass performs 1 to 2 orders of magnitude better than grout. No value was presented for H-3, Cl-36, and I-129 in glass and H-3 in glass because the high-temperature processes will volatilize these elements.
Summary of Intruder Analysis
Results of the intruder analysis are shown in Table V. As mentioned above, the polyethylene post-drilling scenario gives results identical to those for grout. Besides these results, those for the glass waste form and the necklace scenario are also shown. The results show that the glass waste form performs best in the post-drilling scenario. While in the necklace scenario, the waste form holds on tightly to the constituents giveing a greater dose.
Table V. Permissible Waste Concentrations based on Intruder
Scenarios (µCi/m3)
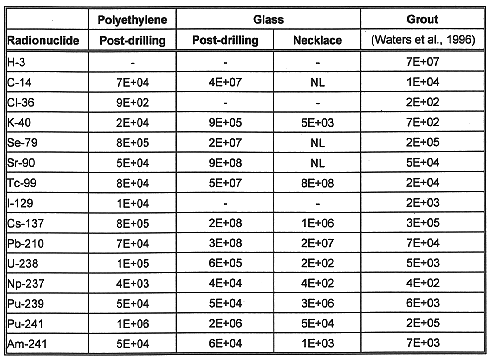
Comparison of Water Pathway and Intruder Results
Table VI summarizes the data from the two previous sections. It lists the waste form that had the highest limiting permissible waste concentration (i.e., the best-performing waste form). It then lists the ratio of that concentration to the concentration for the next best-performing waste form. That is, the most restrictive pathway concentration was found for each waste form and each radionuclide at each site. Whichever waste form had the highest concentration found in this way was deemed the best performing, and the waste form with next highest concentration was considered the second best performing. The waste form geometry and loading are the same as those listed in Table IV.
The data show, not surprisingly, that glass is the best performing waste form for ten of the fifteen radionuclides at both Hanford and Savannah River. For Pu-241 and Am-241, the new necklace intruder scenario restricts the glass performance. Polyethylene was the best performing waste form for four of the radionuclides, Cl-36, I-129, Pu-241, and Am-241. Grout was determined best for H-3 at each site due to the assumption that H-3 would be mostly water and boiled off by the elevated processing temperatures of the other waste forms.
Table VI. Summary of Most Restrictive Pathways

CONCLUSIONS AND RECOMENDATIONS
In general, for releases to groundwater, the glass waste form should perform better than the polyethylene and grout waste forms by up to 3 orders of magnitude. The polyethylene waste form may perform better than the grout waste form for some radionuclides, depending on the selected waste loading. Incorporating the inadvertent intruder analysis into the evaluation makes it clear that no one waste form is best for all wastes at each site. However, an implicit assumption to develop these results is that the waste to be stabilized is compatible with the selected waste form. To provide a context to these relative comparisons, these results should be compared with types of waste and concentrations of radionuclides expected to be present in DOE's treated waste. A comparison of this type could indicate the potential problem areas in terms of waste types and radionuclide combinations. Little research has been conducted on the performance of polyethylene for specific radionuclides. The results presented herein were based on the assumption that all radionuclides performed similarly in polyethylene.
One significant point of discussion at a recent meeting (32) was the intruder scenario requirement and the possibility of relieving shallow land burial sites of this regulatory obligation. The point behind this reasoning is that disposing in hazardous waste cells regulated by CERCLA requires long term institutional controls. Meanwhile, the location of mixed or low-level waste disposal trenches is usually next to the CERCLA sites. The fact that one regulation assumes there will be no institutional controls after 100 years (DOE Order) and the other assumes long term institutional controls is confounding. The outcome of this exercise by the working group meeting referenced is a rewriting of DOE Order 5820.2A which describes the requirements for radioactive material handling. This revision may include steps to make intruder scenario requirements in line with CERCLA.
Costs associated with treatment and disposal also need to be evaluated. The benefits in terms of reduced disposal costs from volume reduction have been estimated elsewhere (33). However, that study assumed a cost model based on fixed unit price for disposal costs that may be applicable for commercial disposal but may not reflect the costs for disposal in a DOE-operated disposal facility, where economic incentives are different. Additionally, that study did not use cost multipliers to account for the stage of development of the treatment technology (34). The cost study should also include the effects of the "transactions costs" (the costs of delays, redesigns, and potential law suits) of bringing a particular waste form to fruition (35). For example, at the Hanford Reservation, site managers are pursuing a vitrified waste form even though its production costs more than that of grout. However, the transaction costs resulting from delays due to stakeholder concerns are expected to be less for the glass than grout waste form.
There are several potential avenues for research that could contribute to the issue of waste form performance in shallow subsurface disposal facilities. These are:
This work was performed at Sandia National Laboratories, under contract to the U.S. Department of Energy, Contract No. DE-AC04-94AL8500.
REFERENCES