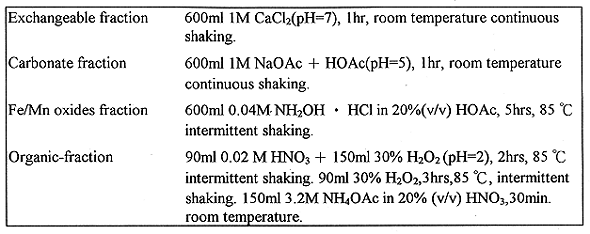
Table I Experimental Extraction Condition Used for 140 Grams
Sediment (dry weight)
Tau-Being Hsu* and Ben-Li Pen
Tamkang University
Department of Water Sources and
Environmental
Engineering
Tamshui , Taiwan , R.O.C.
Lee-Chung Men and Ching-Ho Yang
INER
Chemical
Analysis Division
Lung-Tan , Taiwan , R.O.C.
ABSTRACT
The purpose of this study were to determine the characteristics of the chemical extraction for 60Co and 137Cs radionuclides in the slightly contaiminated drainage sediment by Tessier's sequential extraction and to compare the radionuclides removal using the same solution among three simple processes:shaking method,column washing and microwave treatment.The results obtained show that a significant part of the 137Cs radionuclide was found in the residual fraction of sediment, while the 60Co was mostly associated with Fe/Mn oxides fraction.The removing efficiencies in both microwave treatment and column washing are higher than that in sediment shaking treatment.However,the total removals for all three treatments have almost the same values(~70%). The microwave treatment has advantages of easy operation,time saving and less equipement required.These preliminary experimental results indicated that the microwave treatment might be considered as alterative method in the treatment of heavy-metal contaminated sediments.
INTRODUCTION
The releases of the man-made radionuclides including 137Cs and 60Co to the environment from anthropogenic activities are subject to the different processes, i.e. the atmospheric fallout from nuclear weapons tests and reactors accidents or liquid discharge of the effluent from nuclear reactor. These radionuclides are not permanently bonded to the sediment and soils in most environments, athough the trace elements are rapidly and strongly absorbed by sediments, and may be released by changing the chemical surroundings.Therefore, an understanding of the desorption mechanism of these radiosnuclides is important for sediment managment and treatment.
The sequential extraction have been designed to investigate the distribution of radionuclides throughout the different components of sediments(1~3). The five step sequential extraction scheme of Tessier et al(4) is frequently used although various schemes of fractionation have been develped using different reagents and number of steps(5,6 ). However,sequential extraction is a tedious and time-consuming procedure. In order to overcome long treatment times, numberous studies (7,8) have reported that the use of microwave heating instead of the traditional procedure (shaking at room temperature and heating in a thermostatic bath) in Tessier's extraction scheme.
The aims of this study were(1) to determine the characteristics of the fractionation in the slightly contaminated drainage sediment by sequential extraction,and (2) to compare the radionuclides removal using the same solution (i.e. 0.04M NH2OH.HCl in 20(v/v)HOAc) among three simple treatment processes including shaking method,column washing and microwave treatment.
MATERIALS AND METHODS
The drainage sediment samples were air-dried and sieved at room temperature and the fraction (0.21~0.42 mm)of samples was studied(9).The material was stored in a plastic bottle and mixed by three dimension blender for at least 7 hrs before drawing subsamples. The dry sediment was sequentially extracted according to the Tessier method(4). The fractionations were performed on 140 gram dry sediment in erlenmeyer flask. The extraction condition applied are outlined in Table I.The extracted samples were then centrifuged at 12,000 rpm for 5 min after completing each extraction step and the supernatant was removed for further analysis. The remaining solid were washed with the deionized water and air-dried before analysis for 60Co and 137Cs activities by gamma spectrometry using a Canberra 30% P-type HPGe gamma ray spectrometer. The sample was placed on the top of the detector head and counted for over 50000s.
For sediment treatment, three simple processes were performed to remove metals from the drainage sediment using the same solution (i.e.Fe/Mn oxide extracting solution: 0.04M NH2OH.HCl in 20(v/v)HOAc). For column washing, sediment column were prepared by packing 140 gms of the contaminated sediment in a glass tube, of which the internal diameter was 1.9cm giving a dry density of 1.5 g/cm3.The extracting solution was passed through the samples in the glass tube and then circulated for 5 hrs. The solid sample was separated and air-dried for activity analysis. For shaking method, the extracting solution was added into the contaminated sample and shaked intermittently for 5hrs in the thermostatic bath(85). The solid sample was separated and air-dried for analysis.
For microwave treatment, which was made in open mode, a CEM MDS-81D(600W)microwave oven was employed. The operation steps were1)140g sediment was added to 600ml extraction solution in the vessel,2)placing the vessel on the turnplate of microwave oven, then setting up treatment conditions i.e. only 75% microwave power for 15 mins,3)the solid sample was collected after finishing microwave treatment and dried for g analysis. All reagents were Nacalai Tesque (Japan)analytical grade.
Table I Experimental Extraction Condition Used for 140 Grams
Sediment (dry weight)

RESULT AND DISCUSSION
According to the sequential fractionation scheme of Tessier et.al.(4),metals are categorized as "exchangeable ","bound to carbonates","bound to iron and manganese oxides","bound to organic matter"and "residual" . In this study , we will refer to these fractions as "exchangeable","carbonate","Fe/Mn oxides","organic"and "residual" respectively .Error in the data presented are relatively large due to the low level of radioactivity present in the samples examined.
The distribution of radionuclides in drainage sediment according to Tessier's sequential procedure in which each step was repeated two times are given in Table II . The chemical extraction of the sediment indicated that a significant fraction of the 137Cs was found in the residual fraction while the 60Co radionuclide was mostly associated with Fe/Mn oxides . The 137Cs present in the sample is virtually strongly associated with the residual phase, which is consistent with a number of other study(1,2,10) . However, Cundy and Croudance(2) suggested that varying amounts (40~95) of the 60Co were removed from the estuarine sediment by acetic acid at pH 4.5, corresponding to removal of the carbonate fraction .The removing efficiency of 60Co radionuclide from sediments is better than that of 137Cs.The shaking procedure was repeated four times with fresh extracting solutions for Fe/Mn oxides.Each cycle of shaking was termed a stage. The acitivity amount removed in each stage are listed in Table III. The total removal for 60Co is slightly higher than that from sediment treated twicely, probably due to the redistribution of the element in the sediment . However ,the total 137Cs removal from the sediment treated four times was comparable with that showed in Table II.
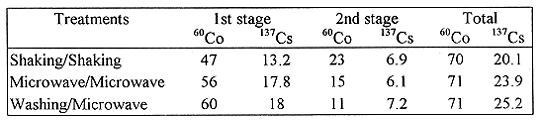
The removing efficiencies of each radionuclide for three treatments in which each treatment included two stages are listed on Table IV. For all three treatments, the radionuclide removal of the first stage is higher than that of the second stage. It is interesting that the removing efficiencies in both microwave treatment and column washing are higher than that in sediment shaking process. However, the total removals for all three treatments have almost the same values(~70%). Compared to shaking method and column washing, the microwave treatment has advantages of easy operation, time saving and less equipment required. These preliminary experimental results indicated that the microwave treatment might be considered as alterative method in the treatment of heavy-metal contaminated sediments.
Table II Results of Sequential Extraction (two times)

Table III Activity Data of Shaking Treatment Process (Bq/KG)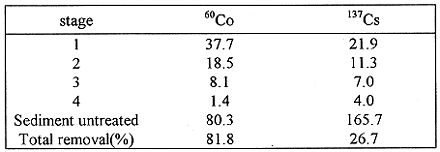
Table IV The Radionuclides Removing Efficiencies (%) of Three
Treatments
REFERENCES
*Address correspondence to this author.E-mail:hsutb@ew001.ew.tku.edu.tw