Table I Batch Equilibrium Test Results
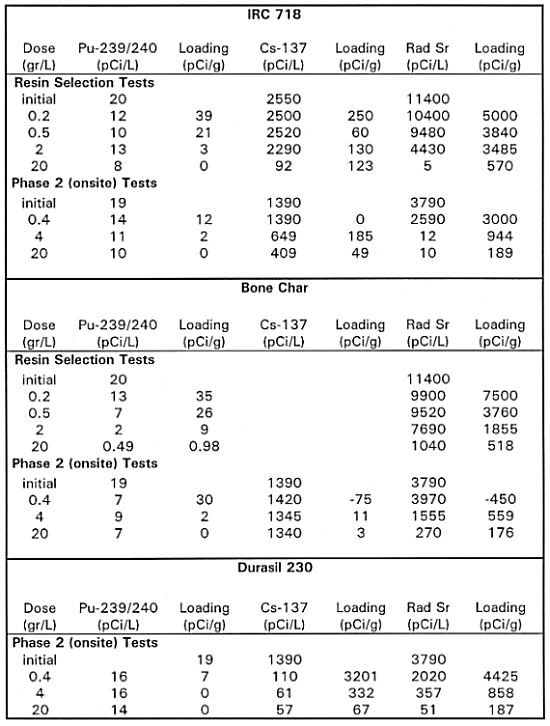
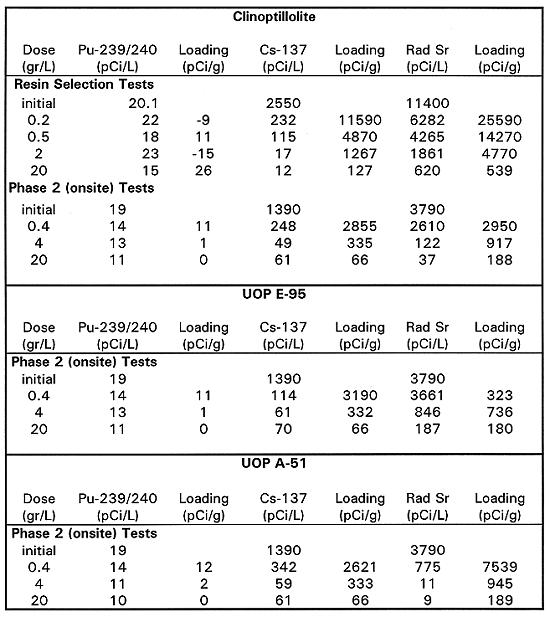
Table II Comparative Performance of Ion Exchange Media Batch
Equilibrium Tests
Abdul Dada
Bechtel Hanford Inc.
David Erb
IT Hanford Inc.
Stuart Shealy
IT Corporation
ABSTRACT
Bench-scale and large-scale pilot treatability testing was conducted in support of remedial design activities in the Hanford Site 200 Area . This testing provided data needed to develop pump and treat systems for the BP-5 Reverse Well groundwater plume in the 200 Area. The treatability testing included bench-scale batch equilibrium (isotherm) and mini-column tests on a number of ion exchange resins and other adsorption media and testing of selected media in the large-scale (100 liters/minute feed) pilot treatment facilities. The mini-column and pilot tests were conducted in the field with groundwater from the BP-5 Reverse well plume.
The B-5 Reverse Well plume contains plutonium, strontium and cesium and a series of batch equilibrium tests were conducted to evaluate several adsorbents and ion exchange materials for removal of these contaminants. Bone Char was effective on plutonium while clinoptilolite, a natural zeolite ion exchange material, was very effective on cesium and strontium. Based on the batch test results, the pilot system included adsorbers packed with bone char and clinoptilolite.
After the batch tests mini-column tests on clinoptilolite were conducted . These tests demonstrated that, while the clinoptilolite has a good loading capacity for both strontium and cesium, 90Sr bleeds through the column fairly rapidly. In addition a second series of batch equilibrium tests were conducted to screen additional ion exchange media for strontium removal. The second round of batch equilibrium tests identified a synthetic zeolite that had better performance for strontium than did the clinoptilolite and also a synthetic resin that was effective on plutonium. The synthetic zeolite was also tested in a mini-column system and gave slightly lower effluent 90Sr than the clinoptilolite. The mini-column and pilot-scale testing also showed that kinetics were limiting strontium removal and that contact times of 40 to 54 minutes will be required for achieving the 8 pCi/Liter criteria for 90Sr. These tests were conducted concurrent with operation of the pilot system.
The pilot-scale testing demonstrated that plutonium and cesium treatment criteria were met for a run length of 3700 bed volumes. Strontium in the effluent rapidly broke through to 1200 pCi/liter, 32% of inlet concentration but did not increase for 3700 bed volumes. One of the mini-column systems, operating with longer contact time with the clinoptilolite, gave better performance, 86% strontium removal for 3500 bed volumes.
This paper summarizes the results of the bench-scale and pilot ion exchange testing for removal of contaminants from the extracted groundwater. A more detailed presentation of these results, as well as discussion of the effect of the pilot-scale pump and treat study on aquifer contaminantconcentrations and other aspects of the pump and treat evaluation, can be found in the 200-BP-5 Operable Unit Treatability Study Report (DOE/RL-95-59, September 1995).
SITE DESCRIPTION
The B5 Reverse Well plume is centered around the B5 Reverse Well, which is located about 305m northeast of the 221B Canyon Building at the Hanford BPlant facility. This well was used for the disposal of medium level radioactive wastes from the Hanford B Plant starting in 1945 and received approximately 303 x 107 L until its abandonment in 1947. Waste liquids were derived from fuel rod dissolution and waste neutralization from the plutonium recovery processes. The wastewater from the B plant was pre-treated to remove radionuclides and the residual waste stream containing reduced levels of cesium, plutonium, and strontium was then allowed to overflow to the ground via the B5 Reverse Well. The B-5 Reverse well discharged the wastewater into the soil column and to the top 3.7 m of the underlying aquifer.
The pilot scale treatability test system was set up in close proximity to the B5 Reverse Well because the plumes were believed to be confined around the reverse well. Based on well testing, well 299E2823, located 1.2 m southeast of the B5 Reverse Well, was chosen to be the extraction well. The effluent groundwater from the system was returned to well 299E287 for re-injection into the existing contaminant plume. Re-injection of treated groundwater into the plume allowed the system to be operated, even when drinking water standards were not met. Re-injection outside of the existing plume could have spread contamination to an otherwise uncontaminated part of the 200 East Area aquifer.
Test Program
Testing for this study was conducted in four distinct phases. The first series of tests, designated as resin selection tests, and were conducted in parallel with the design/construction of the pilot-scale treatment system. Their purpose was to confirm that the resins and other ion exchange or adsorption media selected for the pilot system would provide acceptable removal of the groundwater contaminants of concern. The initial selection had been made based on past results with other waste waters. The resin selection tests included both batch equilibrium tests and one short (1000 bed volume) continuous micro-column test.
The second series of tests were conducted in parallel with the start-up of the pilot system and included an expanded series of batch equilibrium tests designed to screen additional ion exchange media. The goal of these tests was to identify media with better performance than those tested in the resin selection study. Tests of isotopic dilution to increase removal of 90Sr were also conducted.
The third phase of the testing was comprised of a series of continuous mini-column tests that provided additional data to supplement the pilot system data. The mini-columns were 2.5 cm in diameter with approximately one meter of adsorbent. The effect of isotopic dilution and increased residence time on removal of 90Sr was tested. The performance of a synthetic zeolite that showed improved 90Sr removal in the batch equilibrium study was also tested.
The testing in the large-scale pilot treatment system completed this test program.
Batch Equilibrium Tests
Batch equilibrium tests are performed at varying dose rates of adsorbent (generally two or more) to provide equilibrium loading data with which to predict the adsorption of constituents from solution. The models proposed by Freundlich and Langmuir are commonly used. The most commonly used model is the Freundlich adsorption isotherm. Freundlich (1926) proposed that the amount of a solid, or a gas, adsorbed per gram of adsorbing material would be proportional to the equilibrium concentration (for a solid), or the equilibrium pressure (for a gas), raised to some fractional exponent. The Freundlich isotherm, albeit empirical, is used to linearize batch equilibrium data as straight line curves when plotted on log graphs. These curves then can be used to compare the performance of different adsorbents and to estimate saturation loadings and run length for column operation. In the absence of adsorption isotherm plots, batch equilibrium data still provide significant information. In evaluating the results of batch equilibrium tests, the lower dose levels (i.e.,0.2 and 0.4g/L) give a approximation of the loading capacity of an adsorbent. The higher dose levels give an indication of the ability of the same adsorbent to achieve a high quality effluent, assuming kinetics are not a factor (assumes that the adsorbent and the liquid are in equilibrium in the batch test).
Batch equilibrium tests were performed by weighing gram (±0.001g) quantities of different ion-exchange media/adsorbents into 2,000ml polyethylene bottles. Resin/adsorbent doses ranged from 0.2 to 20.0 g/L of groundwater. Two thousand grams of groundwater was added to each bottle. The bottles were then sealed and agitated by rotating tumblers, shaker tables or stirrer for 24h. When the studies were complete, the processed groundwater was decanted or filtered from the ion-exchange medium/adsorbent. The liquid was sent to the laboratory for analysis.
Adsorbents evaluated in the resin selection and onsite batch equilibrium tests are as follows.
Batch Equilibrium Tests - Resin Selection
Resin selection tests were conducted to pre-screen the adsorbents that had been selected for the pilot test and consisted of batch equilibrium and one continuous micro-column tests. IRC718 clinoptilolite (a natural zeolite mineral), and bone char were tested. Based on the results of the resin selection tests, the pilot-scale system was configured with a bone char bed, a clinoptilolite bed and a third mixed bed of these two media.
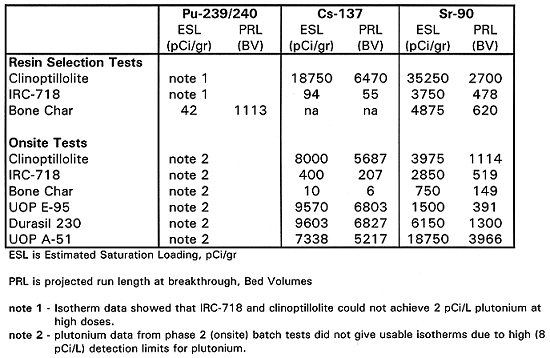
Resin Selection Testing Results - The results of the resin selection and onsite batch equilibrium tests are shown on Table I and Table II. Table I shows the data on strontium, plutonium and cesium adsorption for the various adsorbents, while Table II shows the estimated performance of the adsorbents in column operation. The estimates in Table II are based on the isotherms produced by the data in Table I.
Table I Batch Equilibrium Test Results


Table II Comparative Performance of Ion Exchange Media Batch
Equilibrium Tests

IRC718 is a chelant based cation-exchange resin that has a preference for the heavy metal, multivalent cations. The isotherm data show that this resin had little affinity for cesium and, at the lower doses, strontium removal was poor. Strontium was probably displaced from the resin by other cations (calcium, iron, magnesium, and other metals) that were more strongly adsorbed or present in higher concentrations. Cesium is a monovalent cation that was not expected to adsorb on this resin. In this first round of testing, plutonium loading data were not consistent, and the IRC718 showed no loading capacity at concentrations below 7.7pCi/L, which indicated that meeting the MCL criteria of 1 to 2pCi/L would be difficult with this resin.
Bone char has been used in the 200 Area processing plants to remove plutonium from wastewater and was expected to have some capacity to remove strontium. Two samples of bone char were tested. The bone char did not work very well on strontium or cesium, although it was fairly effective on plutonium.
Clinoptilolite is a naturally occurring zeolite that removes contaminants by both cation exchange and adsorption because of its molecular sieve properties. It was tested on the B5 Reverse Well groundwater. Two sources for clinoptilolite were tested. The American Resources material gave higher loadings and only this data is reported. Clinoptilolite deposits from different mines vary in their composition and adsorptive capacity. The first batch of clinoptilolite, from Resintech, was a high-calcium clinoptilolite. It showed good adsorption for cesium, but tailed off at the high doses and had poor capacity for strontium. A second batch, from AmReC, had a much higher capacity for strontium and better cesium removal at the low concentrations The plutonium isotherm data showed that the AmReC clinoptilolite had poor loading capacity for plutonium.
Table II shows the estimated saturation loadings derived from the batch equilibrium test data. Bone char was the only effective adsorbent for plutonium and gave a predicted 1113 bed volumes until breakthrough. Clinoptilolite had excellent adsorptive capacity for cesium, with a conservatively estimated 6470 bed volume run length. All three adsorbents had useful capacity for strontium but clinoptilolite had the highest loading capacity and predicted run length of 2700 bed volumes. Based on these results, the pilot-scale system was configured with a bone char bed, a clinoptilolite bed and a third mixed bed of these two media.. While the loading capacity of clinoptilolite for strontium at low doses was high (relating to performance at inlet of a column, the residual strontium concentration at high doses (relating more to column effluent) was much higher than the 8 pCi/L drinking water standard. This can sometimes mean early bleed of contaminant in a column effluent. This was demonstrated in a micro-column test described in the following paragraphs.
Clinoptilolite Micro-Column Tests - The microcolumn test used a 1.3-cm (0.5in.) diameter glass column fed by a variable speed tubing pump. The column was packed with 20 ml of clinoptilolite and backwashed with approximately 13 BV of deionized water until the deionized water coming off the top of the column was clear. The column was then allowed to repack before the feed, at 3ml/min, was introduced downflow to the column. The duration of the column study was 1,000BV. During the run, grab and composite samples were taken for analysis to determine effluent quality. These samples were analyzed for total strontium by GFAA. Removal efficiency for total strontium and 90Sr should be equivalent.
The data from this test are shown in Table III and confirmed that low levels of strontium rapidly bleed through the column. Clinoptilolite (in a single stage column with a 7.33 minute empty bed contact time) was not able to get strontium down to the 8pCi/L (0.11ppb) DWS. This test predicted the rapid breakthrough of 90Sr that occurred in the pilot system and additional tests to improve 90Sr removal were planned.
Table III Micro-column Test of Sr-90 Removal
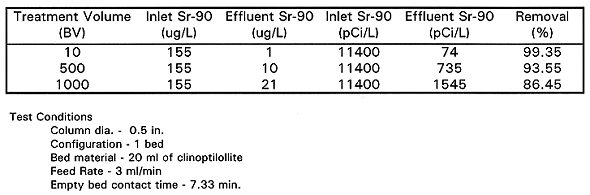
The results of the micro column test also confirmed rapid breakthrough of plutonium and effective cesium adsorption.
Onsite Batch Equilibrium Tests
The second series of batch equilibrium tests (onsite tests) were conducted in parallel with the start-up of the pilot system and included an expanded series of batch equilibrium tests designed to screen additional ion exchange media. The goal of these tests was to identify media with better performance than those tested in the resin selection study. Tests of isotopic dilution to increase removal of 90Sr were also conducted.
For these tests, Isotopic dilution was the addition of non-radioactive strontium chloride to the groundwater in hopes of increasing removal of 90Sr. If 90Sr removal is limited by the equilibrium between the strontium species in the groundwater and the adsorbed strontium on the resin, removal can be improved by adding strontium to decrease the fraction of initial and therefore residual strontium that is 90Sr. (i.e. If groundwater in contact with large amounts of clinoptilolite will always have 0.2 ppb of total strontium in solution and the initial ratio of 90Sr to total strontium is 10,000 pCi/L to 100 ppb, the column will not be able to get below 20 pCi/L. If initial total strontium is increased to 1000 ppb by adding strontium chloride, the residual total strontium will still be 0.2 ppb but the 90Sr will be 2 pCi/L.). The batch equilibrium tests were conducted with the addition of varying amounts (0.2, 2, and 5 ppm) of SrCl2 as a source of nonradioactive strontium. The results of the batch tests and subsequent minicolumn tests showed that isotopic dilution was not effective in increasing 90Sr removal. Adding strontium chloride to the groundwater had no effect on 90Sr loading.
The onsite batch equilibrium test data, relating to comparison of additional adsorbents to the 3 previously tested is shown on Tables I and II. Table I shows the data on strontium, plutonium and cesium adsorption for the various adsorbents, while Table II shows the estimated performance of the adsorbents in column operation. The estimates in Table II are based on the isotherms produced by the data in Table I. Results were consistent with the first round of tests. Bone char was best at plutonium removal. Clinoptilolite was good for cesium and strontium as was UOP A-51, a synthetic zeolite. Two other adsorbents UOP E-95 and Durasil 230 gave performance comparable to clinoptilolite. Clinoptilolite is very inexpensive, compared to the synthetic adsorbents and is the preferred choice for cesium removal.
Flow Through Column Tests (Mini Columns)
To adequately evaluate the effectiveness of an ion-exchange treatment system, it is desirable to obtain multiple sets of performance data. The objective is to process sufficient volumes of contaminated groundwater to load the adsorbent bed to saturation. These data then can be used to obtain the shape of the breakthrough curve, the amount of contaminant(s) that can be removed, and the volume of groundwater that can be processed. When the volume of groundwater processed in either pilot scale or minicolumn tests is converted to bed volumes and the flow rate to bed volumes per unit of time, the data can be directly related, compared, and used to scale up to larger sized systems. For this test, minicolumn data were also intended to supplement data collected from the pilotscale system, since it was anticipated that breakthrough in the pilotscale system beds would not occur within the desired time frame. To achieve breakthrough data more quickly than with the pilotscale system, minicolumns were run 24 h/day. The goals for the mini-column tests are described in the following paragraphs.
From the results of the first group of tests, it appeared that the flow sheet was capable of good removals of 137Cs and 239/240Pu, but 90Sr removals resulted in an effluent that was still higher in concentration than the target MCL of 8pCi/L. The emphasis of the second group of tests focused primarily on 90Sr removal to accomplish the following:
Because microcolumn testing showed that 90Sr would be the most difficult contaminant to remove, the emphasis of both groups of minicolumn tests was to evaluate/enhance the effectiveness of clinoptilolite for the removal of 90Sr.
The results of the minicolumn tests are shown on Tables IV through VI.
Table IV Plutonium - 239/240 Results Mini-column and Pilot-scale
Systems
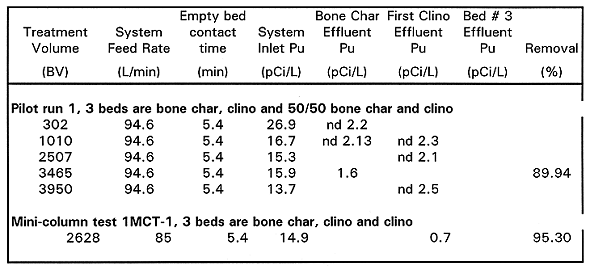
Table V Cesium-137 Results Mini-column and Pilot-scale Systems
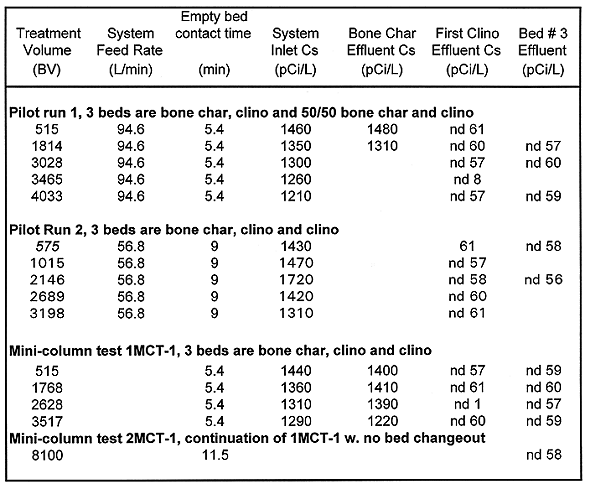
Table VI Strontium-90 Results Mini-column and Pilot-scale Systems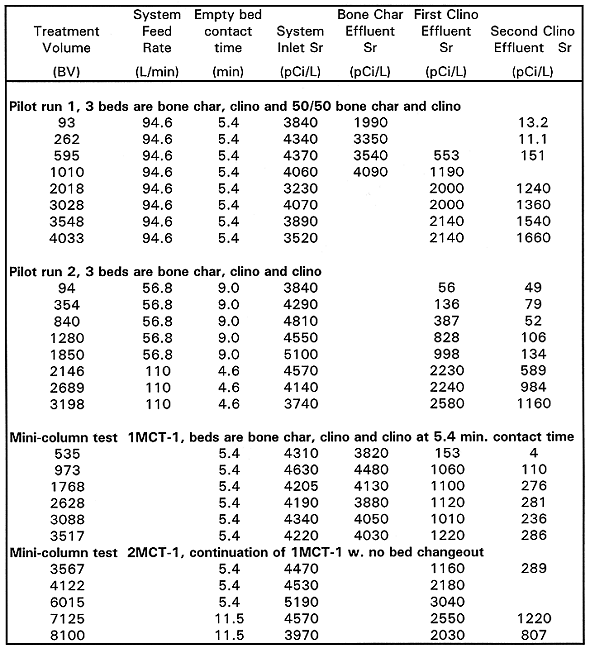
MiniColumn Test Apparatus
The minicolumn test apparatus consisted of a 757 L feed storage tank, a 1-µm prefilter, three (B5Reverse Well site) 2.54-cm diameter by 1.52-m long glass columns, and a 757L effluent collection/storage tank. The filter, columns, pumps, and other components were connected with plastic tubing and fittings. The columns were constructed of heavy wall glass with ground spherical ball joint ends. The adsorbent was added to a depth of approximately 91 cm for a bed volume of ~460ml.
Samples from these tests were primarily analyzed by an onsite laboratory. The detection limits averaged approximately 60pCi/L for 137Cs, approximately 10pCi/L for 239/240Pu, and approximately 4pCi/L for 90Sr. Offsite laboratory results, because of lower background, had lower detection limits and provide the data necessary to show the ability to meet target treatment goals.
MCT1 Results. Column configuration for the 1MCT-1 test was a bone char bed followed by two clinoptilolite columns. MCT-1 run was stopped at approximately 3,500BV, and then resumed for another approximately 4,580BV (total of ~8,100BV). The plutonium data from MCT-1 includes only one data point from the offsite lab. 239/240Pu was below the MCL at 2628 bed volumes. 137Cs was below the MCL for the entire 8100 bed volume run. 90Sr removal was steady at 95% until 3567 until bed volumes where the column plugged with biomass or became saturated. The bone char column contributed to 90Sr removal for the first 500 BV, but most of the removal was provided by the clinoptilolite. This test confirms that clinoptilolite cannot achieve the MCL target effluent of 8pCi/L at short contact times.
MCT2 Results. Column configuration for the 1MCT-2 test was a bone char bed followed by two clinoptilolite columns. This test was done to evaluate the effect of adding a 2ppm spike of SrCl2 to each of the clinoptilolite columns for the removal of 90Sr (BHI 1995). The addition of approximately 1 ppm total strontium in the feed streams to the clinoptilolite columns appeared to reduce the effectiveness of the clinoptilolite for 90Sr removal. The MCT2 lead clinoptilolite column effluent concentrations followed the same trends as that of MCT1 but were consistently about 300 to 400pCi/L higher. The removal efficiencies of the two tests were similar while the bone char was actively removing 90Sr (to 500 BV), but the MCT2 lead clinoptilolite column effluent concentrations increased and stayed consistently higher by approximately 300 to 400pCi/L. Both tests were otherwise run identically.
The addition of SrCl2 did not appear to affect the performance of the clinoptilolite for 137Cs or 239/240Pu removal. Effluent concentrations for both radionuclides were below the detection limit (~60pCi/L 137Cs and ~10pCi/L 239/240Pu) throughout the test.
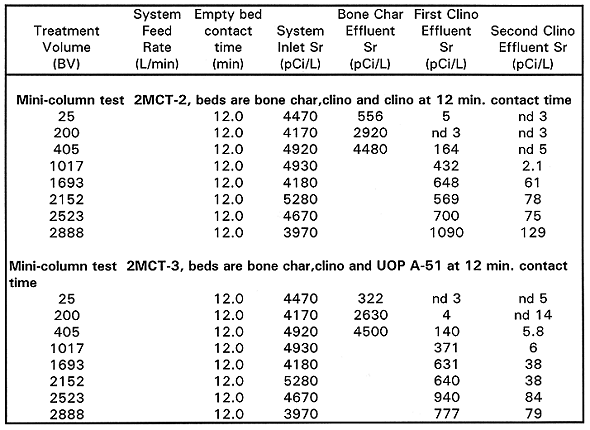
2MCT2 Results. 2MCT2 was run in the same configuration as MCT1 (bone char, clinoptilolite, clinoptilolite), but at a 12 minute contact time per column. The test was run 24h/day for 6 weeks, processing a total of approximately 2,900 BV. The test was operated 24h/day to process the maximum amount of groundwater possible in the 6 weeks of available testing time. The 90Sr data from this test is shown on Table VI.
Since the purpose of this test was to obtain data on 90Sr removal, no 239/240Pu or 137Cs data was collected. For the first 200 BV, the bone char acted as a roughing column for 90Sr. The second clinoptilolite column demonstrated a 90Sr removal efficiency greater than 90% to 1,000BV. Beyond 1,250 BV, it demonstrated an average removal efficiency of 90%. The overall system achieved a 90Sr removal efficiency that ranged from approximately 99.9% through 1,000 BV to 96.8% at 2,900BV. For the first 1,000BV, the effluent concentration was less than the target MCL of 8pCi/L 90Sr. Longer contact time improved 90Sr removals.
2MCT3 Results. 2MCT3 was set up similarly to 2MCT2, except that a UOPA51 column was added at the end instead of clinoptilolite and consisted of bone char, clinoptilolite, and UOPA51 (a synthetic zeolite/molecular sieve) columns. 2MCT3 was run 24h/day for 6weeks. A total of 2,900 BV were processed at a contact time of 12minutes per column. The 90Sr data from this test is shown on Table VI
The bone char again removed a significant percentage of the 90Sr to 200 BV and then became saturated. For the first 200 BV, while the bone char bed was actively removing 90Sr, the lead clinoptilolite bed was removing >99% of its influent (the bone char effluent). Once the bone char became saturated, the lead clinoptilolite bed's efficiency dropped to approximately 90% until 1,250 BV, and then averaged 80% through the end of the test.
The UOPA51 maintained a removal efficiency greater than 90% to the end of test. The last data point indicated a removal efficiency of 89.9%. The system removal efficiency with the UOPA51 adsorbent was greater than 95% for the duration of the test. The overall system effluent concentration was less than the target MCL of 8pCi/L for 90Sr for the first 1,000 BV. The UOP A-51 gave slightly lower 90Sr than the clinoptilolite
Pilot-scale Treatment System
The B-5 Reverse well pilot-scale treatment system consisted of an extraction well, a 31,000L influent storage tank, a skid mounted influent pumping station (with filtration), a skid mounted adsorbent/ion-exchange treatment system, a 31,000L effluent storage tank, a skid mounted effluent pumping station (with filtration), and a return well Groundwater was pumped from well 299E2823 to the influent storage tank. The well was capable of producing at least 132L/min, but the well pump was capable of delivering only 106L/min . Groundwater was pumped from the influent storage tank via the influent pumping station through filters to the ion-exchange treatment system. The treatment system consisted of four downflow columns: three were used as cation adsorber columns for removal of primary contaminants and the fourth for the GAC. Filtered groundwater was pumped to the first column containing bone char for plutonium removal; to the second column containing clinoptilolite for 137Cs and 90Sr removal; then to the third column containing 50% clinoptilolite and 50% bone char as a backup polishing column for all contaminants. The three columns were filled with 1.7m2 of adsorbent, which resulted in a contact time of 5.4 min and a hydraulic surface loading rate of 1.9L/min/m2 in each column at a flow rate of 95L/min . (Bed volume is defined as the volume of adsorbent, 18ft3; contact time is the bed volume divided by volumetric flow rate.) Treated groundwater was transferred to the 31,000L effluent storage tank. The effluent pumping station consisted of a set of pumps and a set of filters to filter the treated water before disposal via the return well.
Pilot Scale Treatment System Results
Two tests were conducted with the pilotscale treatment system at the B5 Reverse Well site, designated as "Run 1" and "Run 2". Because the system was not able to achieve the target 90Sr level after 2 months of operations, run 1 was terminated and the clinoptilolite and mixed bed (clinoptilolite and bone char) media were removed and both beds were recharged with clinoptilolite. The bone char was not changed because it was still demonstrating good 239/240Pu removal. Thus, Run 2 commenced with the original bone char column followed by two columns with new clinoptilolite. The process flow rate was reduced from approximately 95L/min in Run1 to 57L/min to increase the contact time to 8 minutes per column (versus the 5 to 6 min of Run1). Run data from the pilot tests are shown on Tables IV through VI.
A total volume of 2,284,000L, or 4,033BV, of groundwater was processed in Run1. Plutonium removal was good for the entire run. One data point from the offsite lab showed 1.6 pCi/L in the bone char bed effluent at 3465 BV. End of run 239/240Pu was less than 2.5 pCi/L. Cesium in the effluent was below 60 pCi/l. for the entire run. 90Sr rapidly broke through the system and removal slowly declined from 65%b to 50 % from 2000 to 4000 bed volumes. This showed significant adsorptive or loading capacity left on the clinoptilolite beds, despite 90Sr bleeding through the column.
Because the emphasis of Run 2 was 90Sr removal, no plutonium and only limited 137Cs data were obtained. Near the end of Run 2, a tracer test was performed, and the flow was increased to 106 to 114L/min (, which reduced contact time to 5 minutes with a subsequent increase in 90Sr concentrations in the column effluent.
Run 2 concluded after processing approximately 1,448, 559L, or 3,198BV. The system provided an average effluent concentration of 267pCi/L 90Sr and 58.5pCi/L 137Cs. The corresponding removal efficiencies for 137Cs and 90Sr were 96% and 94.5%, respectively. As previously mentioned, no plutonium data were obtained for Run 2.The data from this run show that the longer contact time did improve 90Sr removal. The quality of the effluent from the lead clinoptilolite column decreased over time, with concentrations ranging from a low of 56pCi/L at approximately 100BV to approximately 1,000pCi/L 90Sr at 1,850BV. At 2,150BV, coincident with the onset of the tracer test, the effluent concentration increased to 2,200pCi/L 90Sr. The lead column removed an average of 980 pCi/L over the course of Run 2 for a removal efficiency of 80%. For 137Cs, the removal efficiency was unchanged at 96% from Run 1. The increase in influent concentration and reduction in residence time during the tracer test probably caused some degradation of removal efficiency for the 90Sr.
During the tracer test, a kinetic effect was observed, in that shorter contact times resulted in increased effluent concentrations, increasing to approximately 2,500pCi/L by the end of the test (3,000 to 3,200BV). As mentioned above, longer contact times seemed to show improved 90Sr removals.
CONCLUSIONS
Although this was a very complex study, there are a number of general conclusions that have been drawn from the very significant amount of data that were generated. These are discussed in the following paragraphs. Application of the results of this work to other groundwater or wastewater projects should include a detailed evaluation of the complete data set found in the 200-BP-5 Treatability Study Final Report (DOE-RL-95-59, September 1995).
All of the various batch and column tests showed that 137Cs was readily removed from the groundwater by the clinoptilolite. In particular the mini-column test MCT-1 treated 8100 bed volumes of groundwater, reducing 137Cs from over 1300 pCi/L to less than 66 pCi/L in the effluent from the second clinoptilolite bed. This is well below the treatment criteria of 120 pCi/L. The pilot-scale tests showed less than 60 pCi/L in the effluents for 4033 bed volumes for run 1 and 3198 bed volumes for run 2. The limited data from offsite laboratories indicate that 137Cs levels may have been below 10 pCi/L. The batch equilibrium tests showed that other zeolites have comparable loading to clinoptilolite but no clear advantage, especially in light of the cost differential.
The results for plutonium were also very consistent, although the data were not as extensive, due to the high detection limits for the onsite lab. For the pilot system run 1, the data from the offsite lab indicated that the bone char was effective in reducing effluent 239/240 Pu to non-detectable levels (with a detection limit of 1.5 pCi/L). This is close to the 1.2 pCi/L treatment criteria. Results for the MCT-1 run were comparable.
The 90Sr was quite a bit more problematic than the other radionuclides. The effluent 90Sr for both the pilot and mini-column tests were well above the treatment criteria of 8 pCi/L. This would be discouraging until the effect of residence time or contact time is considered. First order rate constants (K values) were calculated from the pilot scale and minicolumn test data for 90Sr to separate the effect of residence time from bed performance. The rate constants were calculated from the following equation.
Kt = ln(Effluent 90Sr conc./Influent 90Sr conc.)
where K is the kinetic constant and t isthe empty bed contact time. The resulting rate constants for each run are tabulated in Table VII.
Table VII Strontium-90 Results Mini-column and Pilot-scale Systems
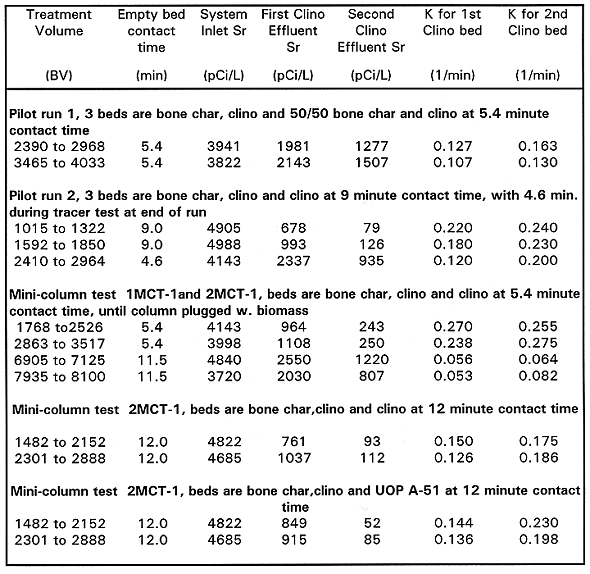
Inspection of the rate constants shows generally good agreement from the first to second bed. The K values are generally higher in the minicolumns relative to the pilot plant. This is probably due to the more efficient contacting in small columns produced by the higher length/diameter ratios and the lower tendencies for small diameter columns to form shortcircuiting channels in the resin.
Based on the K values calculated above, a conservative extrapolation of the data suggests that a three bed system with approximately 18minutes of contact time should allow achievement of 8pCi/L 90Sr in the effluent.
BIBLIOGRAPHY
ANDERSON, J.D., 1990, A History of the 200 Area Tank Farms, WHC-MR-0132, Westinghouse Hanford Company, Richland, Washington.
BARNEY, G.S., K.J. LUECK, and J.W. GREEN, 1992, Removal of Plutonium from Low Level Process Wastewaters by Adsorption, "Environmental Remediation, Removing Organics and Metal Ion Pollutants," ACS Symposium Series 509, American Chemical Society, Washington D.C., pp. 3446.
BHI, 1995a, Risk Based Decision Analysis for the 200BP5 Groundwater Operable Unit, BHI00416, Rev. 00, Bechtel Hanford, Inc., Richland, Washington.
BHI, 1995b, Workplan and Procedures for BenchScale Testing for the 200BP5, Unit #2 Groundwater Operable Unit, BHIOP00042, Rev. 00, Bechtel Hanford, Inc., Richland,Washington.
BHI, 1995c, Workplan and Procedures for Bench Scale Testing of Potential Absorbents for the 200BP5 Groundwater Operable Unit, BHIOP00015, Rev. 00, Bechtel Hanford, Inc., Richland, Washington.
DOERL, 1995a, 200-BP-5 Operable Unit Treatability Test Report Hanford Site, DOE/RL9559, Rev.0 , U.S. Department of Energy, Richland Operations Office, Richland, Washington.
FREUNDLICH, 1926, Colloid and Capillary Chemistry, Methuen and Company, London.
MERCER, B.W., Jr., 1960, The Removal of Cesium and Strontium from Condensate Wastes with Clinoptilolite, HW-66276, Hanford Atomic Products Operations, General Electric Corporation, Richland, Washington.