BENCHMARK ENVIRONMENTAL RADIOACTIVITY AT A RESEARCH
REACTOR SITE
S.R. Bose and R. U. Mulder
Department of Mechanical,
Aerospace and Nuclear Engineering
University of Virginia
Charlottesville, VA 22903
C.A. Sondhaus and D. Silvain
Radiation Control Office
University of Arizona
Tucson, AZ 85724
M. E. Wacks
Department of Aerospace and Mechanical
Engineering
University of Arizona
Tucson, AZ 85721.
ABSTRACT
The assessment of pre-operational benchmark radioactivity in and around a
nuclear facility is necessary to determine whether an observed change in the
radioactivity levels is due to the operation of the facility. A detailed
investigation of radioactivity levels in soil samples obtained from sites at the
University of Arizona (UA) campus and at the off-campus Page Ranch Radioactive
Waste Repository (now closed) was carried out during 1994-95. The on-campus
soils were analyzed to evaluate a potential influence on the radioactivity in
the local environment due to research application of isotopes at various
laboratories at the University and operation of the UA TRIGA reactor.
Unfortunately, no background measurements were made prior to operation of either
facility. In the absence of baseline data, off-campus soils at and near the
repository were analyzed to compare radioactivity in these samples as well as
with the University samples in reference to soil samples collected on the UA
campus.
The variation in natural radioactivity found was attributed to normal ranges
due to soil and rock type affected by natural erosion, weathering and rock
ageing processes. The results indicate that reactor fission or activation
products are not present in the soils obtained from the UA either on- or
off-campus. However, measurable levels of 137Cs fallout were
observed in both on- and off-campus samples. These variations are not related to
the research reactor activities but to natural processes such as rainfall
variations, wind patterns and surface water flow.
INTRODUCTION
Assessment of benchmark local environmental radioactivity is a reasonable
prerequisite for any nuclear facility. The levels of operationally-induced
radioactivity in soils of the area in and around the facility must be monitored
to determine an increase of radioactivity due to facility operation in a timely
manner. Therefore, radioactivity data must be obtained under pre and post
operational conditions, as well as during operation.
The University of Arizona (UA) has a pool-type research reactor (TRIGA), for
which the licensed steady state power is 100 kW. This reactor has been in
operation since December 1958 and is used for instructional reactor operations
(steady state and pulsed experiments) as well as research and development (R&D)
programs. During normal reactor operation, corrosion products of the materials
in the system and trace impurities present in the charging water are activated
and removed in a dual bed ion exchange system. A possible source of mobile
radioactivity are the fission products in the fuel. They may be released during
fuel elements failure in abnormal situations such as dropping an element when
installing it or changing its location.
During normal operation, the soil adjacent to the reactor pit (if the pool
or tank is small) may capture fast and thermal neutrons escaping from the
reactor system. The UA reactor core is 6.4 m distant from the soil below the
reactor vessel. This soil is irradiated by a very weak neutron flux. Therefore,
this soil may contain trace activation products at levels depending on soil
composition, reactor power history and isotopic half-life. Generally, the
isotopes in activated soil are short half-life beta emitters. They do not
present an environmental hazard. Additionally, the local climate is arid and the
structure limits groundwater access. Also, they are present in negligible
concentrations.
The UA owns a closed shallow-land burial site located at the Oracle
Agricultural Center (Page Ranch) in Pinal County, about 45 km from the
University. Different types of radioactive waste were disposed of there by the
Radiation Control Office (RCO) of the UA from 1962 until 1986. In the past, the
RCO collects waste on a monthly basis, and transported it to the burial site via
pick-up and stack-bed truck. This waste included dry waste, liquid waste, animal
carcases, animal wastes and scintillation vials.
The major radionuclides in the waste include 14C, 36Cl,
60Co, 137Cs, 244Cm, 3H, 63Ni,
90Sr, 133Ba, 22Na, 226Ra,
natural U, depleted U, natural Th, 210Pb, and 55Fe. In
addition, the following radionuclides are present in trace amounts: 241Am,
39Ar, 45Ca, 57Co, 134Cs, 54Mn,
65Zn, 109Cd, 204Tl, and 124Pm.
Some low-level activation products (less than 1% of the total activity)
generated by the UA TRIGA facility were also disposed at this site, but due to
their short half-lives, those radionuclides have decayed. The waste was packed
in metal, plastic or cardboard containers and deposited in pits later
backfilled. The soil was compacted by a backhoe passing back and forth over the
pits.
Over the years 1962-1986, UA generated about 1426 m3 of
low-level radioactively contaminated wastes. The total activity of these wastes
in January 1996 was estimated (1) at about 1.1x10 4 mCi. Of this
total activity, the contributions of 3H and 14C were 79%
and 20%, respectively. The remaining radionuclides constituted only 1% of the
total activity.
The disposal site is in a mesquite-grasses-cholla ecosystem. The climate is
semi-arid with low rainfall, high evapotranspiration, and low humidity. Rain
occurs as thundershowers in July and August that are characteristically of high
intensity and short duration, and as rains in December and January which are
gentler, of longer duration, and more prone to infiltration. The disposal site
rests on 240-270 m of alluvial deposits consisting of unconsolidated and
semi-consolidated clay, silt, sand, and gravel.
Whether radioisotopes originate from artificial or natural sources, they may
eventually reach society through different pathways depend upon isotropic
half-lives, release conditions and transfer mechanism. Human exposure is
classified as either internal or external. To cause internal radiation exposure,
radioisotopes deposited in soils, water, vegetation, aquatic species etc., must
enter the human body via ingestion of food, or through inhalation of dust.
External exposure results from gamma-ray radiation emitted by radionuclides
present in soils and building materials. Both internal and external exposures
are caused by a combination of natural and artificial sources.
Among the artificial sources of isotopes, 131I, 90Sr
and 137Cs are recognized as the most important. Strontium-90 and
137Cs, which are generally present in the environment as a result of
fallout (weapon testing), are biologically important because of their i) high
yield in the fission process (5.76% and 6.13% respectively), ii) long physical
(about 30 years) and biological half lives, and iii) easy entry into the human
body. Iodine-131 has a short half-life (T1/2= 8 day) and is a
problem only for a short period (perhaps 3 months) after release. Iodine is
accumulated in the thyroid and is of concern only in the event of serious
nuclear accidents concurrent with fuel damage and fission product release.
The natural sources of radioactivity in the environment are generally
classified into two groups; primordial and cosmogenic. The present investigation
involves the primordial group, which includes 40 K and a majority of heavy
elements isotopes belonging to the three naturally occurring decay series of
238U, 235U and 232Th. Environmental
radioactivity is due principally to the presence of these series of nuclides in
the earth, either because their half-lives are comparable to the age of the
earth or because they are being produced continually through the decay of
unstable parent nuclides. Isotopes in the 238U and 232Th
series are comparably more important because the 238U and 232Th
abundances are significantly higher than the 235U abundance. Uranium
is a mixture of three isotopes consisting of 238U (99.3%), 235U
(0.7%) and a trace quantity of 234U. The specific activity of 238U
in natural U is 12.2 x 10-3 Bq kg-1. Thorium-232 is
present as 100% of natural Th, and has a specific activity of 4.0 x 10-3
Bq kg-1. In the course of time, both 238U and 232Th
reach near radioactive equilibrium with their respective daughters (2).
Uranium may enter the food chain via plants, at levels depending mainly on
the soil matrix, plant metabolism and 238U availability. Uranium-238
daughters play a significant role in the exposure of man and his environment
through naturally occurring radioisotopes. For example Radium-226 is chemically
similar to Ba or Ca and is easily absorbed from soil by plants. Thus, the main
sources of environmental "contamination " from the U and Th series are
due to diffusion of radon gas into the atmosphere in living and work places and
other series decay products formed at the time of daughters decaying. Certain
areas of the world, such as the monazite sand beaches of Brazil and India, have
background radiation levels on the order of 10 times the average worldwide
background (3-5) due to the high U and Th content of the sand.
The naturally occurring 40K isotope is radioactive, having a
half life of 1.3 x 109 years; its abundance in natural K is 0.0118%
(6), imparting a specific (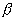 -)
activity of about 30 Bq kg-1K. Because of sufficient abundance and
energetic beta emission (1.3 Mev), 40K is the predominant
radioactive component in everyday foods and in human tissues. It is, therefore,
responsible for most of the internal radiation dose. Since
40K is ordinarily present in all soils and emits a high energy
gamma-ray (1.46 Mev), it also contributes about one third of the external
radiation dose to man from natural background sources.
-)
activity of about 30 Bq kg-1K. Because of sufficient abundance and
energetic beta emission (1.3 Mev), 40K is the predominant
radioactive component in everyday foods and in human tissues. It is, therefore,
responsible for most of the internal radiation dose. Since
40K is ordinarily present in all soils and emits a high energy
gamma-ray (1.46 Mev), it also contributes about one third of the external
radiation dose to man from natural background sources.
A detailed study was carried out during 1994-1995 to investigate the levels
of natural and fallout radioactivity in soils obtained from sampling sites at
and near the University of Arizona (UA) campus and at the off-campus Page Ranch
(the closed radioactive waste repository). The UA soils were examined for
increased radioactivity due to UA reactor operation. The Page Ranch soils,
obtained from the waste area as well as from a nearby reference site, were
compared similarly to see if the operation of the sites had affected the local
radioactivity of the environment. Samples from all sites were collected four
times a year, in the months of January, April, July and October for two years,
in order to assess possible seasonal variation of activity levels. Levels of
activity due to Th daughters, U daughters, 40K and 137Cs
in the soil samples of the above locations were determined and compared.
MATERIALS AND METHODS
Sample Collection and Preparation
Soil samples were collected from the following on-campus locations:
Engineering Building, Harvill Building, Football field, Franklin Building and
Graduate Library of the University of Arizona (Fig. 1a). In addition, samples
were collected also from the Page Ranch waste area, which is located about 48 km
north of the University of Arizona campus (Fig. 1b). The Page Ranch repository
is located within the University of Arizona's Oracle Agricultural Center and is
situated at an elevation of about 1.5 km above sea level while the University is
at an elevation of 1 km above sea level with Public access to the facility being
restricted. The repository area is about 152 m by 30 m in size, and is
surrounded by a fence to limit access to authorized personnel.
Surface soil samples were collected randomly from different points within
the repository on a routine basis. Reference soil samples were collected from
the east side of the repository. All soil samples were collected at the same
time of the year from a depth of about 0 to 60 mm and processed for measurement
under identical conditions. The samples were air dried at atmospheric
temperature, ground, stored, weighed and sealed in aluminum cans, and then
counted using an HPGe detector. The aluminum cans used had a holding capacity of
approximately 425 g of soil or ~425 ml of water, and were supplied by the
Central State Can Co, U.S.A. Soil sample dry mass in the cans was adjusted to
425 g for all measurements.
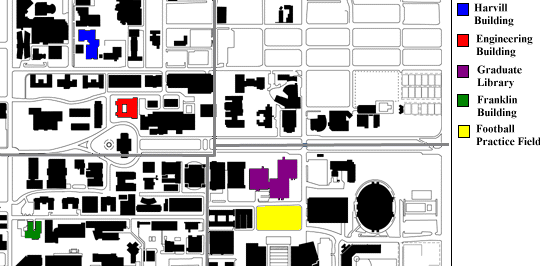
Fig. 1a. Soil sampling sites of the
University of Arizona.
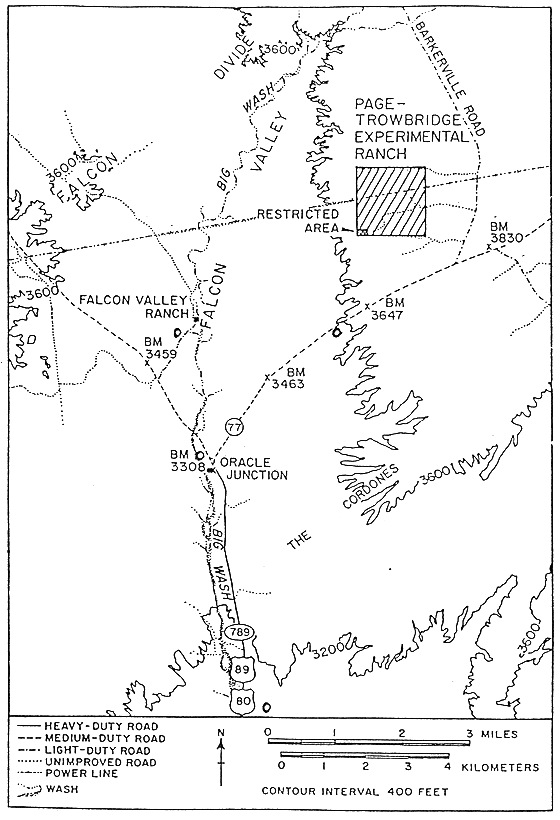
Fig. 1b. Soil sampling sites of the
Page Range Radioactive Waste Repository.
Determination of Gamma-ray Activity
Each 425 g sample of dried soil contained in an aluminum can was "counted"
using a shielded HPGe detector for about 15 h. For measurement of sample
background, an aluminum can containing 425 ml of distilled water was counted for
15 h, and the spectral analysis was made under identical conditions used for the
soil sample analysis. The background count of each water blank sample was
subtracted from the collected soil sample count to obtain the true count rate
due to radionuclides present in the sample. All count data were obtained using
an energy calibrated detector. In order to determine counting efficiency of the
detector, a standard reference sample (7) was counted in the HPGe detector under
identical geometrical conditions. The counting efficiency of the detector as a
function of gamma energy is shown in Fig. 2.
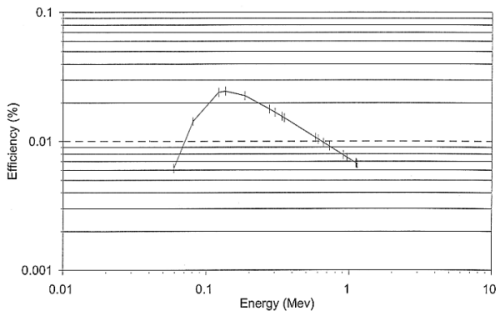
Fig. 2. Detector (HPGe) efficiency as
a function of gamma-ray energy.
Detailed information about equipment and operating instructions may be
obtained from the Instruction Manual (8). The spectral analyses of the count
data were made using the program "GDRMAIN" version 4.6 provided by
Quantum Technology (9). The spectrometric analysis was verified using a
different program, "PCA-II", obtained from Oxford Instruments (10).
The prominent gamma-ray energies observed in the spectra for different
radionuclides are presented in Table I. All the net counting rates were
converted into specific activity (Bq kg-1) using the efficiency data
of Fig. 2.
Table I Relevant Prominent Gamma-ray Energies
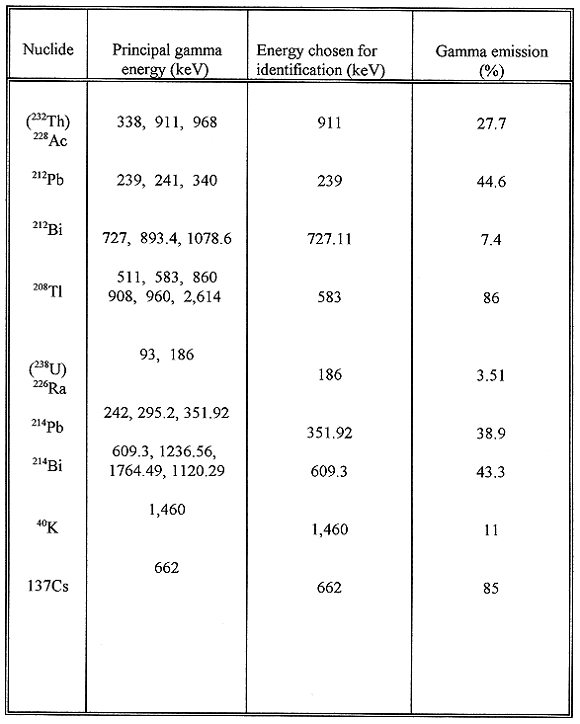
Counting Errors
The counting errors for individual radionuclide were calculated at a (±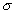 ) standard deviation, and the
values are expressed as percentage of the net counting rates. The average
activity for each radionuclide was calculated using the total number of samples
employed for the analysis. The error for the average value was obtained by
dividing the total error (total ) for each radionuclide by the total number of
samples. The Below Detection Limits (BDL) values for all the isotopes were
calculated in accordance with the standard literature formula (11).
) standard deviation, and the
values are expressed as percentage of the net counting rates. The average
activity for each radionuclide was calculated using the total number of samples
employed for the analysis. The error for the average value was obtained by
dividing the total error (total ) for each radionuclide by the total number of
samples. The Below Detection Limits (BDL) values for all the isotopes were
calculated in accordance with the standard literature formula (11).
The counting errors associated with the measurement of 228Ac,
212Bi, 212Pb and 208Tl ranged from 1.6% to
2.2% (average 1.9% ), 2.8% to 4.5% (average 2.9%), 0.5% to 1.0% (average 0.7%),
and 1.0% to 1.7% (average 1.2%), respectively. The error for 232Th
activity computed from the total error () for each radionuclide ranged form 1.5%
to 2.0% with an average of 1.8%. The counting error on U daughters were observed
to be 3.0% to 4.0% (average 3.8%) for 226Ra, 0.5% to 0.9% (average
0.7%) for 214Pb, and 0.9% to 1.2% (average 1.0%) for 214
Bi. The error for 40K had a range of 19% to 21% with an average of
20%. For 137Cs the counting error was found to vary from 0.1% to
0.6% with an average of 0.4%.
RESULTS AND DISCUSSION
The levels of activity for each radionuclide including its counting error
(%), and BDL (Bq kg-1) values for different radionuclides in the
soil samples collected at the UA Engineering Building, Harvill Building,
Football Field, Franklin Building, Graduate Library and Page Ranch area during
1994-95 are presented in Table II. The BDL values for a counting time of 54000
sec for 228Ac, 212Bi, 212Pb, 208Tl,
226Ra, 214Pb, 214Bi, 40K and
137Cs were estimated to be 9, 18, 4, 7, 17, 4, 5, 91 and 0.70 Bq
kg-1, respectively. The observed levels of radioactivity are discussed below:
In accordance with the Th decay series (12), the activity of 232Th
may be determined from daughters 228Ac, 212Bi, 212Pb
and 208Tl. The Th daughters are assumed to be in secular equilibrium
in the decay chain (13-15). It has also been reported (16) that the thorium
daughters in any disturbed soil reach near-secular equilibrium if the soil is
stored in a closed container for at least one month after collection. All
samples in this measurement were stored in aluminum cans. The cans containing
dry soils were sealed and counted after a minimum of one month's storage.
Therefore, the daughter activity should provide a good basis for an
approximation for the activity of the parent 232Th. The contribution
of 208Tl is about 36% of its parent 212Bi in the decay
series. On the basis of this assumption, the measured activity of 208Tl
needs to be multiplied by 2.78 in order to get the activity of its immediate
parent 212Bi (15,16).
Table II Moisture Content (%) in the University of Arizona on and
off Campus Soils Collected During 1994-95
The direct measurement of 226Ra in soil is difficult. The
gamma-ray emission of 235U and 226Ra are at 185.75 keV
and 186.10 keV, respectively. The energy resolution of the HPGE detector (full
width at half maximum) used for the measurements was in the range of 2 keV.
Therefore, it was not possible to discriminate these isotopes spectrometrically.
The measurement of activity in the 186.10 keV spectrum included the activity of
both 226Ra and 235U. The use of the 186.10 keV line for
the measurement of 226Ra may lead to an error of about 25% in the
226Ra activity due to the presence of the 185.75 Kev line of 235U.
Therefore, all the 226Ra data were corrected by assuming a 25%
contribution of 235U (16), which introduces an additional
uncertainty of about ± 1% in the 226Ra determination.
The activities of 214Pb and 214Bi, are relatively
easy to measure without any interferences. They are daughters of 226Ra,
with abundant gamma emissions. In undisturbed soils, the short lived daughter
products of 226Ra are expected to be in secular equilibrium with
226Ra. Since the half life of 222Rn is 3.82 days, it may
be assumed that in disturbed soils, a considerable amount of 222Rn
will escape, once it is produced in the decay chain, into the environment by
diffusion through the soil. This 222Rn diffusion disturbs the
equilibrium ratios between 214Pb and 214Bi in the decay
chain. As a result, secular equilibrium among the U daughters in the decay
series, i.e, the daughters after 226Ra, may not exist. In a medium
of undisturbed surface soils, however, the activity of 238U is
reported to be in secular equilibrium with its daughter 226Ra
(16,17). It has been reported that 222Rn and decay products normally
remain trapped in the crystal lattice structure of the soil matrix, and
equilibrium is only disturbed through chemical processes such as acid digestion
or heating (18). Van Cleef (1) in 1994 reported that the activity of 226Ra
can be measured by the determination of its daughters 214 Pb and
214 Bi. Therefore, measurement of the activity of 226Ra
in an undisturbed soil can provide an estimate of the activity of 238U
in the same sample. The activity levels of different radionuclides in the
samples collected from different UA on- and off-campus locations are discussed
in the next section and presented in Table III.
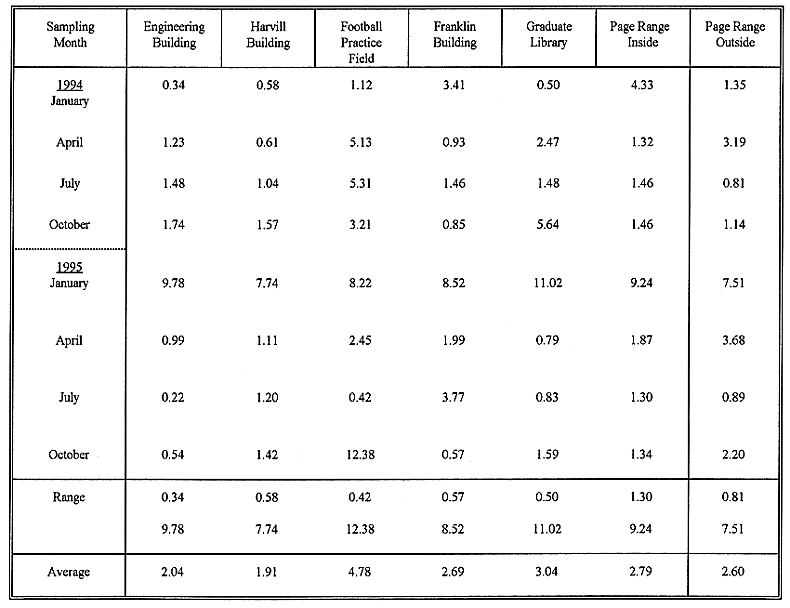
Table III Radioactivity (Bq kg-1 dry soil ± percent error) in
the University on and off campus soils collected during 1994-95
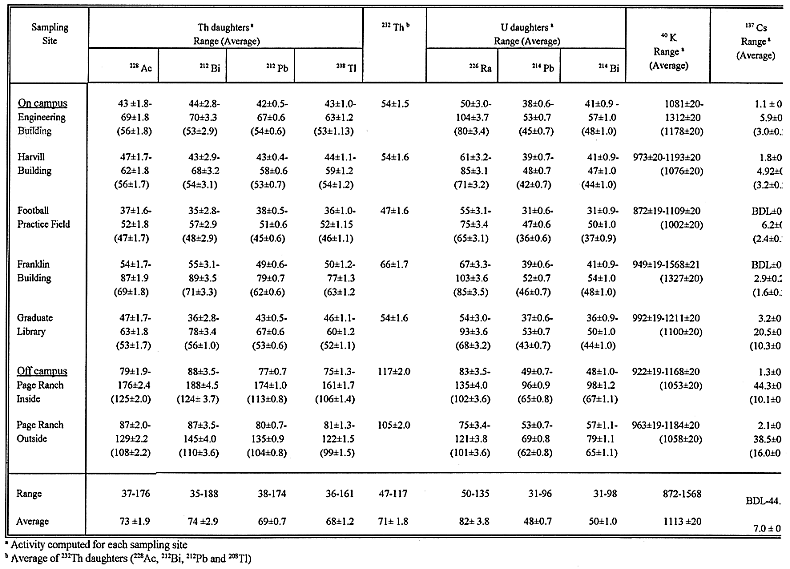
Activity of Thorium Daughters
The observed activity levels of 228Ac, 212Bi, 212Pb
and 208Tl in the UA on- and off- campus soils range from 37 ±
1.6 to 176 ± 2.4 Bq (average 73 ± 1.8 Bq), 35 ± 2.8 to 188 ±
4.5 Bq (average 74 ± 2.8 Bq), 38 ± 0.50 to 174 ± 1.0 Bq (average
69 ± 0.7 Bq), and 36 ± 1.0 to 161 ± 1.7 Bq (average 68 ±1.2
Bq) kg-1 dry soils. The level of 232Th (i.e. the average
activity of the Th daughters), ranges from 47 ± 1.6 to 117 ± 2.0 Bq
with an average of 71 ± 1.7 Bq kg-1 dry soil. When the on- and
off- campus soils were analyzed separately, the following results were obtained.
For on-campus soils, the activity of 232Th ranges from 47 ± 1.6
to 66 ± 1.7 Bq with an average of 55 ± 1.6 Bq kg-1 soil.
In the case of off-campus (i.e. Page Ranch) soils, the activity ranges from 105
± 2.0 to 117 ± 2.0 Bq with an average of 111 ± 2.0 Bq kg-1
dry soil. It is observed that the activity of 232Th for off-campus
soils is higher by a factor of two than that of on-campus soils, probably due to
different mineral forms in the soils. The literature survey shows that the
levels of 232Th in the UA soils compare well with those reported for
Virginia (16) (range 5 to 84 Bq kg-1 dry soil) and Louisiana (20)
(range 11 to 62 Bq kg-1) soils. The activity of 232Th in
Spanish soils as measured from the 911 keV peak of 228Ac was
reported (21) to be widely variable, ranging from 5 to 254 Bq with
an arithmetic mean value of about 41 Bq kg-1 soil. This average
value is comparable to those observed in the UA on-campus soils, but lower than
the off-campus (Page Ranch) soils. The world average concentration of 232Th
was reported to be 25 Bq kg-1 dry soil (22). In our study, the
observed results indicate that the levels of 232Th are higher than
the world average by a factor of about two for on-campus soils, and a factor of
four for off-campus soils, respectively.
Activity of Uranium Daughters
The soil activity data indicates that 226Ra was not in secular
equilibrium with its daughters. Therefore, the average activity of Ra daughters
may not represent well the activity of 238U. The activity of 214Pb
and 214Bi averages about 60% of the activity of 226Ra.
Since 226Ra is close to its parent 238U in the decay
chain, the activity of 226Ra may provide an estimate of the activity
of 238U (16,17). The activity of 226Ra in the UA soils
ranges from 50 ±3.0 to 135 ±4.0 Bq with an average of 82 ± 3.8 Bq
kg-1 dry soil. On the basis of on- and off-campus soils, the
concentrations of 226Ra range from 54 ± 3.0 to 103 ± 3.6
Bq (with an average of 74 ± 3.3 Bq kg-1) for on-campus, and 75 ±
3.4 to 135 ± 4.0 Bq (with an average 102 ±3.6 Bq kg-1) for
off-campus soils. The activities of 214Pb and 214Bi in
the UA soils range from 31 ± 0.6 to 96 ± 0.9 Bq (average 48 ± 0.7
Bq), and 31 ± 0.9 to 98 ± 1.2 Bq (average 50 ± 1.0 Bq) kg-1
soil, respectively. The observed range of 226Ra, 214Pb,
and 214Bi activities (range 8 to 310 Bq, average 51 Bq) kg-1
soil appear to be consistent with those reported for surface soils (2,
13,14,23). In comparison to the world average (22) (30 Bq kg-1) the
levels of 226Ra in on- and off- campus soils are higher by factors
of two and four, respectively.
Natural 40K Activity
The levels of 40K in different sampling sites of the UA range from 872 ±
19 to 1568 ± 20 Bq kg-1, with an average of 1113 ± 18 Bq
kg-1 dry soil. Since the activity of 40K in both on- and off-campus
soils shows a similar trend, no clear interpretation regarding differences in
activities between these sites can be made. However, some variation of activity
levels among different sampling sites was observed. From the average result, it
may be seen that the 40K activity in the soils obtained from the Franklin
Building site appears to be higher than those obtained at other sampling sites.
The reason for such variation may be attributed to the particle size of K
bearing minerals, soil weathering conditions, soil compositions, and
leaching/migration of K containing particles due to rainfall. The observed data
compare well with previously reported values in the literature (16,21). The
world average activity of 40K is reported (22) to be 370 Bq kg-1,
which is about three times lower than the average result of our observation.
Fallout 137Cs Activity
The activity of 137Cs in the UA soils varied widely, having a
range of BDL ± 0.1 to 44.2 ± 0.6 Bq with an average of 7.0 ± 0.4
Bq kg-1 dry soil. In general, the activity in the samples of Page
Ranch and Graduate Library are higher than those obtained in the rest of the
sampling sites. It may be seen that the highest level of 137Cs was
obtained in the reference sample (Page Ranch reference), and the lowest in the
sample obtained from the Franklin Building site. The average results indicate
that 137Cs activity within the Page Ranch radioactive waste
repository is lower than that observed in the reference site (Page Ranch
reference (outside)). The level of 137Cs in the sample obtained from
the site of Engineering Building is very similar to those observed in other
on-campus sites, except for the location of the Graduate Library where the
sample registered a higher activity. The deposition of fallout 137Cs
on soil surface depends on atmospheric wind direction and rainfall. Cesium is a
monovalent element and is quite soluble. Therefore, the variation of 137Cs
levels among different sampling sites is mainly due to variation of
concentration levels of this element in the atmospheric air, and its ultimate
fallout on the neighboring ground soils by precipitation and rainfall. The
observed results compare well with the 137Cs levels (range BDL to 71
Bq kg-1 surface soils) reported earlier (2,14,17,24).
CONCLUSION
The soil samples collected from the vicinity of the UA Engineering Building,
during the period of January 1994 - January 1995 did not show any fission or
activation products. The results for 137Cs clearly indicate that
there is no 137Cs impact on the on-campus soils due to operation of
the UA TRIGA reactor, although a detectable level of fallout 137Cs
was observed in both on- and off-campus soils. These levels of 137Cs
activity are most probably the result of atmospheric fallout due to nuclear
weapon tests (25-30) . The results indicate that secular equilibrium among the
thorium daughters exists in all soil samples considered for this study and that
the assumption of secular equilibrium between U and its decay products cannot be
made. The reason for this inconsistency cannot readily be explained without
further detailed studies. In order to satisfy this point a separate study, which
was not the main purpose of the present investigation, may eventually be carried
out. The activity of Th and U daughters, on the average, is observed to be
higher in the off-campus, Page Ranch, than the on-campus soils.
The results of the overall study may be used as reference for assessment of
environmental radioactivity in the areas surrounding the on-campus TRIGA
reactor, as well as the off-campus radioactive waste repository. The on-campus
information will be needed before eventual decommissioning of the reactor, and
will also be useful in the event of actual or alleged dispersal of radionuclides
from the reactor.
ACKNOWLEDGMENTS
Dr. John Williams, Professor, of the UA NEE Department, kindly supported the
principal author during this work in summer of 1994. Dr. Williams made many
valuable suggestions useful for the completion of this study. His time, advice
and financial support is deeply appreciated. The assistance of the RCO staff in
the completion of this work is also gratefully acknowledged.
REFERENCES
- Burial History Report. University of Arizona, Radiation Control Office,
Tucson, Arizona (1962-86).
- S.R. BOSE, "Radioactivity in Central Virginia Sediments and Soils",
Master of Science Thesis, University of Virginia, Charlottesville, Virginia May
(1988).
- Academia Brasileira de Ciencias. International Symposium on Areas of High
Natural Radioactivity, Rio de Janeiro (1977).
- K.B. MISTRY, A.R. GOPAL-AYENGAR, and K.C BHARATHAN, "On the
Radioactivity of Plants from the High Radiation Areas of the Kerala coast and
Adjoining Regions ll", Studies on the uptake of alpha and gamma emitters.
Health Phys. (1965).
- E.PENN-FRANCA, M.FIZZMAN, LOBAO., H. TRINADADE., C.COSTA-RIBEIRO, and
P.L.SANTOS,. "Radioactivity in the Diet in the High Background Areas of
Brazil", Health Phys. (1970)
- D.T. GOLDMAN, "Chart of the Nuclides (7Th Ed.)", Knolls Atomic
Power Laboratory operated by the G.E. Company under direction of Naval Reactor,
USAEC (1964).
- Custom Mixed gamma standard, serial No. A1297. North American Scientific,
Inc. 7435 Greenbush Ave, North Hollywood, CA 91605 (1993).
- Instruction Manual, Model TC 244. Tennelec, Inc. Oak Ridge Turnpike, Oak
Ridge, Tennessee 37831-2560, U.S.A. (1985).
- Gamma Spectrometry Data Reduction Software, Quantum Technology, 830
Franklin Court, Marietta, Ga 30067 (1986).
- Oxford Instruments, Inc. Nuclear Measurement Group, P.O.Box 2560, Oak
Ridge, Tennessee 37831 (1993).
- International Atomic Energy Agency. Measurement of radionuclides in foods
and the environment. Technical report series No.295, Vienna, IAEA (1989).
- Radiological Health Handbook. U.S. Department of Health and Education and
Welfare, Public servive, Rockville, Maryland 20852 (1970).
- T.E. MYRICK, B.A. BERVEN, F.F. HAYWOOD, "Determination of
concentration of selected radionuclides in surface soils in the U. S",
Health Phys. (1983).
- A.S. MOLLA, M.M. AHMAN, and S.R. HOSAIN, "Distribution of gamma
emitting radionuclides in soils of the Atomic Energy Research Establishment,
Savar, Bangladesh", Health Phys. (1986).
- S.R. JOSHI, "Nondestructive determination of selected U and Th series
radionuclides in biological samples". Health Phys. (1987).
- S.R. BOSE, T.G. WILLIAMSON, R.U. MULDER, M.A. MOLLA, "Rob. Impact of a
2 MWth research reactor on radioactivity in sediments", Health Phys.
(1993).
- U.C. MISHRA, S. SADASIVAN, "Gamma spectrometric measurement of soil
radioactivity", Int. Journ. Appl. Radiat. Isot. (1971).
- B. KAHN, R. ROSSON, J. CANTREL, "Analysis of 228Ra and 226Ra
in public drinking water supplies by a gamma-ray spectrometer", Health
Phys. (1990).
- D.J. VAN CLEEF, "Determination of 226Ra in soil using
214Pb and 214Bi immediately after sampling", Health
Phys. (1994).
- J.R. MERIWETHER, S.F. BURNS, R.H. THOMPSON, J.N. BECK, "Evaluation of
soil radionuclides using geological based sampling techniques", Health
Phys. (1995).
- L.S. QUINDOS, P.L. FERNANDEZ, J. SOTO, C. RODENAS, J. GOMEZ, "Natural
Radioactivity in Spanish Soils". Health Phys. (1994).
- United Nations Scientific Committee on the Effect of Atomic Radiation.
Sources, effects, and risks of ionizing radiation. Report to the General
Assembly, with Annexes. New York: United Nations (1988).
- YU-MING LIN, PIC-HUO LIN, CHING-JIANG CHEN, CHING CHUNG HUANG, "Measurement
of terrestrial gamma radiation in Taiwan", Health Phys. (1987).
- E.C. MALCOLM, L. BARRY, FRAKHAUSER, "Distribution of fallout 137Cs in
Hawaii", Health Phys. (1984).
- H.F. HUNTER, N.E. BALLOU, "Fission product decay rates",
Nucleonics (1951).
- R.C. BOLLES, J.E. BALLOU, "Calculated activities and abundances of
235U fission products", USAEC Report, U. S. Naval Radiological Defense
Laboratory Report, USNRDL; (1956).
- R.C. MANDEVILLE, "Slow neutron induced activities", Nucleonics
(1951).
- N.H. SHIPMAN, "Detection of 54Mn in radioactive fallout", Science
(1972).
- P.O. STROM, "Long lived cobalt isotope observed in fallout",
Science (1958).
- W.E. LIBBY, "Radioactive fallout particularly from Russian",
October series. Proc. Nat. Acad. Sci. 1 (959).
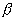 -)
activity of about 30 Bq kg-1K. Because of sufficient abundance and
energetic beta emission (1.3 Mev), 40K is the predominant
radioactive component in everyday foods and in human tissues. It is, therefore,
responsible for most of the internal radiation dose. Since
40K is ordinarily present in all soils and emits a high energy
gamma-ray (1.46 Mev), it also contributes about one third of the external
radiation dose to man from natural background sources.
-)
activity of about 30 Bq kg-1K. Because of sufficient abundance and
energetic beta emission (1.3 Mev), 40K is the predominant
radioactive component in everyday foods and in human tissues. It is, therefore,
responsible for most of the internal radiation dose. Since
40K is ordinarily present in all soils and emits a high energy
gamma-ray (1.46 Mev), it also contributes about one third of the external
radiation dose to man from natural background sources.




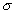 ) standard deviation, and the
values are expressed as percentage of the net counting rates. The average
activity for each radionuclide was calculated using the total number of samples
employed for the analysis. The error for the average value was obtained by
dividing the total error (total ) for each radionuclide by the total number of
samples. The Below Detection Limits (BDL) values for all the isotopes were
calculated in accordance with the standard literature formula (11).
) standard deviation, and the
values are expressed as percentage of the net counting rates. The average
activity for each radionuclide was calculated using the total number of samples
employed for the analysis. The error for the average value was obtained by
dividing the total error (total ) for each radionuclide by the total number of
samples. The Below Detection Limits (BDL) values for all the isotopes were
calculated in accordance with the standard literature formula (11).

