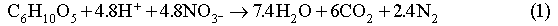
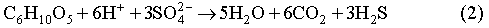
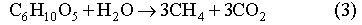
Yifeng Wang
Sandia National Laboratories
WIPP
Chemical & Disposal Room Department
MS 1341, P. O. Box 5800
Albuquerque, NM 87185-1341.
tel: (505) 848-0896, fax: (505) 848-0881
Laurence H. Brush
Sandia National Laboratories
WIPP
Chemical & Disposal Room Department
MS 1341, P. O. Box 5800
Albuquerque, NM 87185-1341
Richard Vann Bynum
SAIC, Inc.
Albuquerque, NM 87106
ABSTRACT
A large quantity of organic materials will be emplaced in the waste isolation pilot plant (WIPP). Those material will potentially be biodegraded in the presence of liquid water in the repository, producing a large amount of CO2. The accumulation of CO2 in the repository will 1) acidify the brine, through the dissolution of carbon dioxide and the dissociation of carbonic acid in the brine, and 2) increase the concentrations of carbonate species capable of forming stable complexes with actinides in the solution. Those detrimental effects, however, can be effectively mitigated by adding MgO backfill. The MgO reactions will practically remove all CO2 from both gaseous and liquid phases and buffer the brine pmH within a desired range, thus significantly improving the WIPP long-term performance.
INTRODUCTION
The Waste Isolation Pilot Plant (WIPP), located in a salt bed in southern New Mexico, is designed by U.S. Department of Energy to demonstrate the safe and permanent disposal of design-basis transuranic waste. WIPP performance assessment requires consideration of radionuclide release in brines in the event of inadvertent human intrusion. The mobility of radionuclides depends on chemical factors such as brine pmH (-log molality of H+) and CO2 fugacity. According to current waste inventory estimates, a large quantity (~ 109 moles C) of organic materials will be emplaced in the WIPP (DOE/CAO, 1996). Those organic material will potentially be degraded by halophilic or halotolerant microorganisms in the presence of liquid water in the repository, especially if a large volume of brine is introduced into the repository by human intrusions. Organic material biodegradation will produce a large amount of CO2, which will acidify the WIPP brine and thus significantly increase the mobility of actinides. This communication addresses 1) the rate of organic material biodegradation and the quantity of CO2 to be possibly generated, 2) the effect of microbial CO2 production on overall WIPP performance, and 3) the mechanism of using MgO to mitigate this effect.
MICROBIAL CO2 PRODUCTION
The occurrence of microbial gas generation in the repository will depend on: 1) whether microbes capable of consuming the emplaced organic materials will be present and active; 2) whether sufficient electron acceptors will be present and available; 3) whether enough nutrient swill be present and available (Brush, 1995). Apparently, all these factors can not be evaluated accurately based on our current knowledge. Because of these uncertainties, we are not able to either eliminate the possibility of the occurrence microbial gas generation or propose that microbial gas generation will certainly occur in the repository. In order to bracket all the possibilities, we assume that only half realizations will have significant microbial gas generation in WIPP performance assessment calculations.
The biodegradation of organic materials follows the sequence of the following reaction pathways, according to the order of decreasing energy yield per carbon atom in each reaction (Berner, 1980; Brush, 1995; Wang and Van Cappellen, 1996):
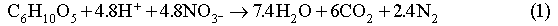
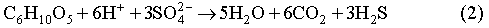
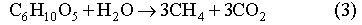
We ignore the reaction pathways of aerobic respiration, dissimilatory Mn(IV) and Fe(III) reduction, since the quantities of O2, Mn(IV) and Fe(III) initially present in the repository will be negligible relative to the other electron acceptors. In Reactions (1) through (3), biomass accumulation is also not taken into account. This is because significant biomass accumulation seems unlikely in the WIPP repository and the accumulated biomass, if any, will be recycled by microbes after all biodegradable cellulosics is consumed.
Cellulosics, plastics, and rubber are identified as major components of organic materials to be emplaced in the WIPP (DOE/CAO, 1996). Cellulosics is much more biodegradable than the other components and its biodegradation rates have been experimentally measured under anticipated WIPP conditions (Francis and Gillow, 1994; Francis et al., 1997). The rate of cellulosics biodegradation was determined by incubating representative cellulosic materials (filter paper, paper towels, and tissue) in WIPP brine with microbes enriched from various WIPP environments (Francis & Gillow, 1994; Francis et al., 1997). The incubation experiments were conducted under various conditions: aerobic or anaerobic, inundated or humid, with or without bentonite, and amended or unamended with nutrients or NO3-. Because the repository is expected to become anoxic shortly after waste emplacement and also because bentonite will not be added with the waste according to the current waste emplacement plan, the experimental data from anaerobic incubation without bentonite present are most relevant to expected WIPP conditions. Considering that the current experimental data are mostly for denitrification (Reaction 1), but not sulfate reduction (Reaction 2) and methanogenesis (Reaction 3) (Francis & Gillow, 1994; Francis et al., 1997), we assume that the range of denitrification rate can apply to sulfate reduction and methanogenesis.
CO2 production data is used to estimate the rates of cellulosics biodegradation, for two reasons: 1) there are experimental data available on the CO2 dissolution in WIPP brine (Telander and Westerman, 1997) and, therefore, it is easy to correct the CO2 production data for gas dissolved in the brine; 2) since cellulosics biodegradation did not reach the stage of methanogenesis in the experiments, according to Reactions 1 and 2, the consumption of one mole carbon of cellulosics will produce one mole of CO2. This 1:1 relationship is independent of oxidation state of carbon in cellulosics. Therefore, it is rather straightforward to determine the amount of cellulosics biodegraded from the amount of CO2 produced.
Experimental data show a strong dependence of CO2 generation on the concentrations of nutrients and nitrate (Fig. 1). The maximum CO2 generation was observed in nitrate- and-nutrient-amended samples. In those experiments, after a short lag phase, CO2 first linearly increased with time and then approached some limiting value as its production rate diminished (Fig. 1). If we assume that biodegradation is nitrate- or nutrient-limited, the experimental data can be explained by Michaelis-Menton kinetics (Chapelle, 1993). Michaelis-Menton kinetics, which describes the dependence of microbial reaction rate on substrate concentration, can be expressed by:
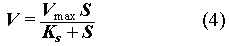
where V is the microbial reaction rate; Vmax is the maximum value of the rate; Ks is the concentration of the limiting substrate; is a constant. Eq. (5) states that the microbial reaction rate becomes independent of the substrate concentration, if the latter is high enough, i.e. S >> Ks and V = Vmax. In this circumstance, the reaction product accumulates linearly with time before the substrate is sufficiently depleted. In other words, in our cases, the linear part of CO2 vs. time curve gives the estimate of the maximum rate of cellulosics biodegradation.
From Figure 1 and with a correction for CO2 dissolved in the brine in the experiments, we estimate the maximum and minimum rates of cellulosics biodegradation under inundated conditions to be 0.3 and 0.01 mole C/kg/year, respectively. The maximum rate is estimated from the data obtained from both NO3- and nutrients-amended experiments, whereas the minimum rate is derived from the data obtained from the inoculated-only experiments without any nutrient and NO3- amendment. Under humid conditions, experimental data show no clear correlation between CO2 production and nutrient concentration (Francis et al., 1997). The best estimate of the maximum rate of cellulosics biodegradation under humid condition is 0.04 mole C/kg/year. The minimum of the humid biodegradation rate is set to 0, corresponding to the cases where microbes become inactive due to nutrient and water stress. Under brine-inundated conditions, at the maximum rate, all organic materials will be biodegraded in ~ 200 years.
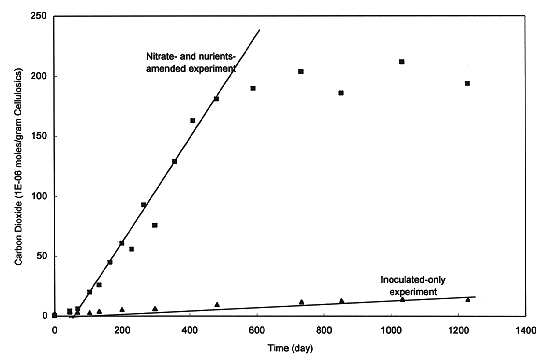
Fig. 1. Amount of carbon dioxide
accumulated in the head space in anaerobic and brine-inundated cellulosics
biodegradation experiments (Francis et al., 1997).
The rates of plastics and rubber biodegradation under expected WIPP conditions were measured by Francis et al. (1997). The experimental data show that plastics and rubbers are much less biodegradable than cellulosics, although the data themselves are not sufficient for us to constrain the long-term biodegradation rate for plastics and rubbers. There are two factor that may potentially increase the biodegradability of those materials: long time scale and cometabolism. Over a time scale of 10,000 years, plastics and rubbers may change their chemical properties and therefore their biodegradability. Cometabolism means that microbes degrade an organic compound but do not use it as a source of energy or of their constituent elements, all of which are derived from other substrates (Alexander, 1994, p. 177-192). In the WIPP repository, plastics and rubbers, which are resistant to biodegradation, may still be cometabolized with cellulosics and other more biodegradable organic compounds. Because of these uncertainties, we recommend a 50% chance for the biodegradation of plastics and rubbers in the event of significant microbial gas generation for the WIPP performance assessment. We further suggest lumping plastics and rubbers into cellulosics and applying the ranges of cellulosics biodegradation rate to plastics and rubbers. This treatment is conservative in respect of repository pressurization and actinide solubility.
Based on the inventory estimates for nitrate (2.6x107 moles) and sulfate (6.6x106 moles) (DOE/CAO, 1996), the fractions of individual biodegradation pathways (Reactions (1) through (3)) are estimated to be: denitrification - 4%, sulfate reduction - 2%, and methanogensis - 94%. Note that methanogenesis produces one half mole of carbon dioxide per mole of organic carbon consumed. With total organic carbon inventory of 109 moles C, we estimate that about 5x108 moles of CO2 will be generated in the repository, if organic materials are completely biodegraded.
IMPACT OF CO2 PRODUCTION ON REPOSITORY CHEMISTRY
Although the actual CO2 partial pressure in the repository is not easy to estimate without consideration of detail mass transfer between the repository and its surrounding geologic media, a simple thermodynamic calculation, based on the real gas model developed by Duan et al. (1992) for a pure CO2 system, indicates that the CO2 partial pressure can reach the lithostatic pressure, if all CO2 generated by microbial reactions is contained in the repository. The accumulation of CO2 in the repository has two significant impacts on WIPP brine chemistry: (1) It will acidify the brine, through the dissolution of carbon dioxide and the dissociation of carbonic acid in the brine, and (2) it will increase the concentrations of carbonate species capable of forming stable complexes with actinides in the solution. Therefore, overall, the microbial production of CO2 in the repository will significantly enhance the mobility of actinide in the brine release.
Table I Major Element Composition of the Representative WIPP Brines
(Brush, 1990, p. 20-22)
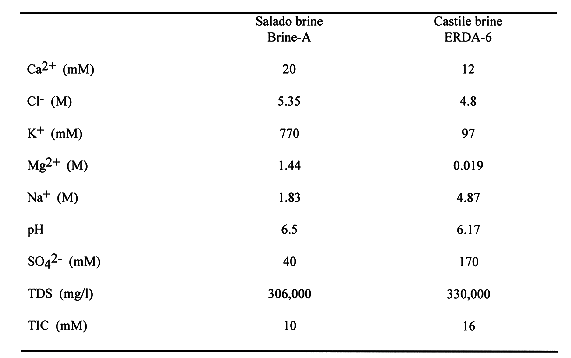
According to the human intrusion scenarios developed in WIPP performance assessment, in which boreholes will possibly penetrate the repository or the brine pockets in Castile formation below the repository, two types of brines will be involved in radionuclide release after a human intrusion: Salado brine and Castile brine. The former, represented by brine A, is derived from the geologic formation where the repository is located, whereas the latter, represented by brine ERDA-6, is derived from the brine pockets in the geologic formations below the repository. The composition of the two brines is listed in Table I (Brush, 1990). The pH of the original brines is close to neutral values. In order to study the brine pH changes as the repository becomes pressurized with CO2 gas, a reaction-path model calculation has been conducted with the computer code EQ3/6 v. 7.2a (Wolery, 1992; Wolery and Daveler, 1992). In the calculation, we have assumed that the CO2 dissolution and dissociation in the brine is the main factor causing brine acidification. The calculation shows that the brine pmH can be as low as 4.5 and the CO2 fugacity can be as high as 55 atm, if the repository is pressurized with CO2 up to the lithostatic pressure of ~ 150 atm (Fig. 2). Under such chemical conditions, the solubility of actinides in the brine is expected to be considerably high.
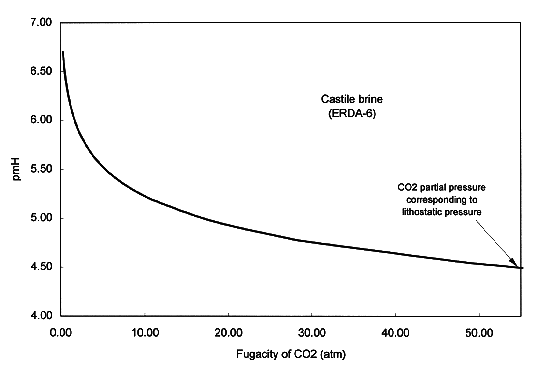
Fig. 2. The pmH of Castile brine
(ERDA6) as a function of CO2 fugacity.
CHEMICAL CONTROL
In order to mitigate the detrimental effect of microbial CO2 generation on the WIPP performance, a sufficient amount of MgO (2x109 moles) will be added to the repository as a backfill. MgO will react with brine and CO2 through the following reactions:
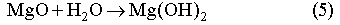
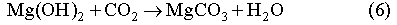
Reaction (6) will buffer CO2 fugacity (fCO2) at ~ 10-7 atm. This low CO2 fugacity implies that Reaction (6) will practically remove all CO2 from both gaseous and liquid phases. The mineral formation and brine pH resulting from MgO reactions have been calculated with computer code EQ3/6 v. 7.2a. The calculation shows that the addition of sufficient MgO into the repository will buffer pmH at 9.2 for Salado brine and at 9.9 for Castile brines (Fig. 3). Under those chemical conditions, actinide solubility becomes minimal (Novak and Moore, 1996). Therefore, MgO backfill can effectively mitigate the effect of microbial CO2 generation and control the repository chemistry within a desired range.
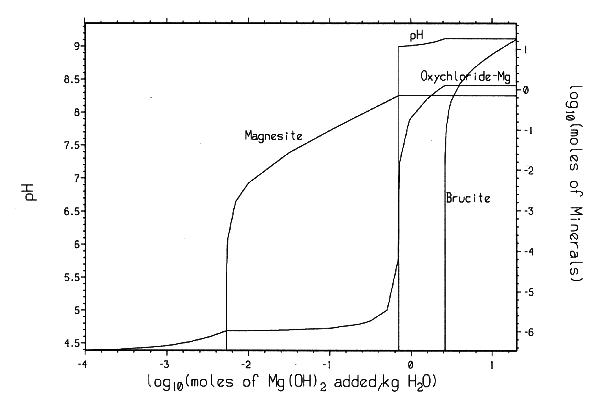
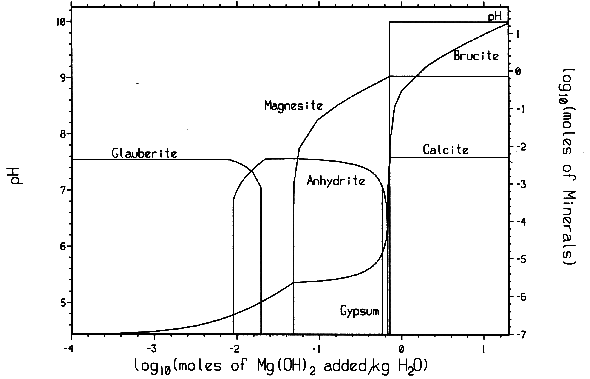
Fig.
3. Mineral formation and pmH changes as Mg(OH)2 is added to WIPP
brines: (A) Brine-A and (B) ERDA-6.
The EQ3/6 v. 7.2a calculation indicates that the mineral equilibrium between Mg(OH)2 and MgCO3 constitutes a chemical invariant point. At this chemical invariant point, the brine pH is essentially determined, e.g. for the Castile brine, by the following equations describing reaction equilibria and charge balance:
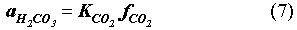
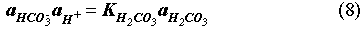
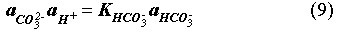
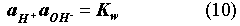
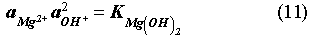
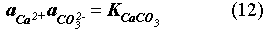
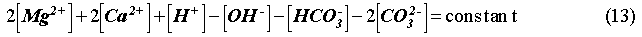
where ai is the activity of chemical species i; Ki is the equilibrium constant of a dissociation or mineral dissolution reaction i. Note that fCO2 in Eq. (7) is constant and fixed by the equilibrium constraint on Reaction (6). Thus, Eqs. (7) through (13) constitute a closed system for [H+]. A similar set of equations can be written for the Salado brine. The above equations show that the brine pmH depends on the activity coefficients of the involved chemical species, the charge balance constant appearing in the right hand side of Eq. (13), and which stable mineral phases form. This is why the MgO reactions give rise to different pmH values for Castile and Salado brines, although the fCO2 remains the same for both cases.
The presence of Ca(OH)2 as cementious materials in the waste can also affect WIPP brine pmH and fCO2. A thermodynamic calculation shows that a sufficiently high ratio of Ca(OH)2 to water can shift the chemical system from the chemical invariant point of Mg(OH)2/MgCO3 to the chemical invariant point of CaCO3/Ca(OH)2. At the point of CaCO3/Ca(OH)2, the brine pmH is buffered at 13 for Castile brine and 12 for Salado brine. However, based on the current inventory estimate for Ca(OH)2, which is about 8x106 moles, the overall possibility for the WIPP chemistry to be controlled by the chemical invariant point of CaCO3/Ca(OH)2 is low. This is because the amount of Ca(OH)2 present in the waste is relatively small and it can be easily consumed by reaction either with microbially generated CO2 or with MgCl2 in Salado brine. Considering that actinide solubility is higher at the chemical invariant point of Mg(OH)2/MgCO3 (Novak & Moore, 1996), we recommend using this actinide solubility for the WIPP performance calculations.
CONCLUSION
A large quantity of CO2 may be generated by the microbial degradation of organic materials in the WIPP. The microbial production of CO2 in the repository will significantly enhance the mobility of actinide in the brine release. This detrimental effect can be effectively mitigated by adding MgO backfill. The MgO reactions will practically remove all CO2 from both gaseous and liquid phases and buffer the brine pmH within a desired range, thus significantly improving the WIPP performance. Also, because the MgO reactions will maintain constant chemical conditions over the whole 10,000 year regulatory time period, adding MgO reduces uncertainties in the prediction of actinide mobility for the long-term WIPP performance assessment.
REFERENCES