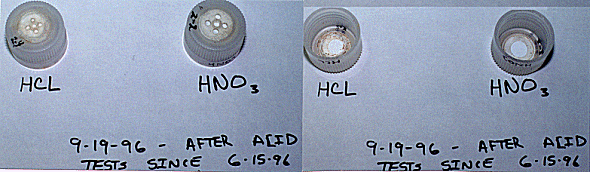
Fig. 1. shows the top surface and the
interior surface of caps exposed To HCL and HNO3 for more than 90
days
Terry J. Wickland
Nuclear Filter Technology, Inc.
William V. Conner
Los Alamos Area Office, Rocky Flats
Plant
ABSTRACT
At the Rocky Flats Plant draining process pipes and tanks that contain as much as 140 grams per liter of plutonium in aqueous hydrochloric acid and nitric acid solutions pose a challenge for interim storage. Solubilized plutonium solutions stored for up to six years in pipes and tanks are drained into one-gallon polyethylene bottles for interim storage until permanent solidification or immobilization may be conducted. Until the solutions are treated, they are stored in one-gallon, low density or high density polyethylene bottles capped with threaded polypropylene tops. At the Rocky Flats Plant, experience has shown that radiolytic effects, specifically, hydrogen gas generation, pressurizes the bottles which accelerates embrittlement of the polyethylene bottle.
Nuclear Filter Technology has developed a low cost method of safely venting the hydrogen gas from the poly-bottles through a membrane, which is permeable to vapors yet impermeable to liquids. Pressure equalization caps (PEC's) allows hydrogen gas diffusion, yet retention of liquids in the event that the bottle is capsized. The membrane is thermally fused to the interior surface of the polypropylene cap. With the thermally bonded membrane, the pressure equalization cap is physically durable, and chemically resistant to the severe acid conditions of the liquid waste. The PEC's have undergone extensive testing including acid compatibility tests to verify vent integrity after exposure to hydrochloric acid (HCL), nitric acid HNO3 and mixtures of both acids for periods of 40 days and longer. The PEC's allow the safe release of gas that may be generated in the storage of solubilized uranium or plutonium, yet will retain liquid contents in the event that the bottle is capsized. The membrane also filters out greater than 99.97% of sub-micron particulates which is verified by challenging the membrane with a stream of DOP aerosol and measuring filtration efficiency with an optical photometer.
The established performance criteria for the PEC's are that each vent, after 40 days of exposure to 6.0 Normal nitric, hydrochloric or 50/50 mixture of each acid, must prove to be leak tight (no liquid release) for a period of 8 hours, given a liquid head pressure of 0.92 psi.
Test results demonstrate that the membrane and the sealing mechanism of thermally fusing the membrane to the polypropylene cap is durable and tolerant of the acidic vapors. It has been demonstrated that the PEC's will withstand liquid head pressure of 0.92 psi, for periods of 8 hours and longer, even after 40 days of exposure to the acids. Further, each cap was tested for liquid release at a pressure of 7 PSI after six months of exposure to the acids. None leaked during the pressure test which lasted for 6 hours.
INTRODUCTION
At the Rocky Flats Plant, nearly 10,000 gallons of acidic plutonium solutions exist in process pipes and tanks that must be drained. Presently, the liquid waste consists of up to 140 grams per liter of solubilized plutonium in various concentrations of nitric acid, hydrochloric acid and nitric-hydrochloric acid mixtures. The waste liquid is drained into one-gallon high density polyethylene bottles for interim storage until more permanent treatment or immobilization may be performed. Since the liquids contain up to 140 grams of solubilized plutonium, alpha decay produces hydrogen gas that rapidly pressurizes the polyethylene bottle. A secondary problem is that the polyethylene bottles eventually become brittle due to the combination of pressurization, excess hydrogen and the acidic contents.
Nuclear Filter Technology developed a pressure equalization cap (PEC) utilizing a membrane thermally fused to the interior surface of the polypropylene cap. The technique of thermally fusing the membrane to the interior surface of the cap proved the only feasible method of securing the membrane to the perforated cap. A review of existing commercial adhesives indicated that there was no known adhesive that would adequately bond between the polypropylene substrate and the (expanded Teflon, PTFE) membrane, especially given the adverse environment of hydrochloric and nitric acids. A review of existing technology indicates that a Gortex membrane, thermally bonded to a substrate such as a polypropylene cap is novel, and, as a result, a patent application has been filed.
QA TESTING
After assembly, each cap is leak tested using a 46" column of water. Experience has shown that if there is not a positive seal made when fusing the Gortex to the cap, this pressurized leak-test identifies the leak immediately. In production, a similar pressure test will be used to verify integrity of each cap made.
Pressure equalization caps, used on poly- ethylene bottles, storing solubilized plutonium, solution increases the useful life of the bottle. The membrane, which is permeable to gases, yet impermeable to liquids allows hydrogen to diffuse at 6.8 E-06 mole/ mole fraction/ second, which is well above the TRUPACT-II minimum requirement of 1.9 E -6 mole/ mole frac/ sec. A secondary function of the pressure equalization vent is that it assures ambient pressure within the polyethylene bottle. The vents also remove 99.97% of 0.3 to 0.5 micron particles when challenged with DOP aerosol.
ACID COMPATIBILITY TESTS
There were three phases of development and testing of the pressure equalization caps. It was only after the first two attempts at producing an acceptable vented cap, that an adequate means to conduct pressure tests of the caps was designed. A summary of the first two phases is described next.
PHASE-I
During the first phase of acid compatibility testing, from February 15, 1996 to May 21, 1996, it was found that the cap that had been exposed to HCL failed the pressure test. After close examination of the cap and the seal, it was decided that the cap actually had a poor seal in it prior to testing. Indeed, the caps were not tested as carefully during the first phase of development as they were in later stages. The cap exposed to the 50/50 mixture of 6 normal HCL and HNO3, and the cap exposed to HNO3, passed the leak test.
PHASE-2
The second phase of testing began on June 15, 1996 when three PEC's were prepared, and then subjected to acid compatibility tests. Prior to testing, each cap was filled to its top with water to determine if it was leak tight. The three samples, identified as #2-1, #2-2 and #2-3 were then exposed to acid mixtures for a minimum of 42 days. After 42 days of exposure, each cap was tested again for leak tightness at the test height of 26" water column. The PEC #2-1, exposed to the mixture of nitric and hydrochloric acid, failed the leak test. After inspection with a microscope of the interior surface of the cap, it was decided that the cause of the failure was likely due to a very slight scar in the center of the membrane. It was also likely that the condition existed prior to the start of acid testing. At a low head pressure, i.e. 6" of water, the cap did not leak, but at the test pressure of 26", it leaked very slowly. The imperfection in the cap existed prior to acid testing. As a result, it was decided to start a new series of caps, this time however, each cap would be leak tested using 46" water column. Although initial photographs of the caps were not taken, Fig. 2 shows the top surface and interior surface of the caps exposed to HCL and HNO3 after more than 90 days of contact. Neither of the caps showed any signs of degradation or discoloration. Also, each cap was pressure leak tested and no leakage occurred.

Fig. 1. shows the top surface and the
interior surface of caps exposed To HCL and HNO3 for more than 90
days
PHASE-3
For the third phase of development, the column was fabricated to test each cap at 46" water column, nearly 1.6 PSI, in order to assure a leak tight seal. With the subject cap threaded securely to the fitting at the bottom, a valve is opened and the water column pressurizes the cap seal. When pressure testing prior to the acid compatibility test, the liquid height was set at 46" water column. The test height after acid compatibility tests is adjusted to 26 inches water column.
Six pressure equalization caps were prepared by thermally fusing a membrane to the interior surface of the polypropylene cap. The membrane was affixed such that the non-woven polyester backing was on the clean side, and the expanded PTFE faced the acid solution. The caps were threaded onto a bottle containing the designated test acid mixture. After allowing the caps to vent the bottles for a minimum of 40 days, each cap was tested for water entry at a pressure of 0.92 PSI (26" water Column). None of the caps showed adverse degradation after compatibility testing, and none experienced water entry at the specified pressure. Photographs of the PEC's were taken prior to the start of, and after the acid compatibility tests. Figure 2 below shows PEC's top and interior surface.

Fig. 2. Shows PEC's # 3-1, 3-2, 3-3,
3-4 top surface prior to acid compatibility tests
EXPOSURE TO HYDROCHLORIC ACID AND NITRIC ACID
After the required 40 days of exposure to the test acid, each cap was removed and examined closely. Notes were made as to the general appearance of the cap. Next, each cap was photographed.
The caps that had been exposed to 6 normal HCL or HNO3 showed no signs of degradation and only slight discoloration consistent with normal aging and dirt accumulation from being in a laboratory. Figure 3 shows caps #3-1 and #3-2, after more than 40 days of exposure to HCL and HNO3.
The caps that had been exposed to HNO3 showed some signs of a yellowish/ brown discoloration. No other signs of degradation were observed. It is plausible that the discoloration was actually on the polypropylene cap or the polyester backing and not on the membrane.
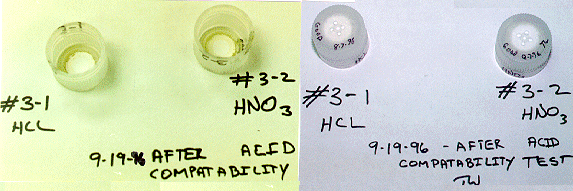
Fig. 3. Shows the top Surface and the
interior surface of PEC # 3-1 after 42 days of exposure to HCL, and PEC #3-2
after more Than 41 days exposurer to HNO3
EXPOSURE TO A MIXTURE OF 50/50 6 NORMAL HYDROCHLORIC AND NITRIC ACID
The caps exposed to the mixture of 50/50 6 normal HCL and HNO3 showed no signs of degradation and only normal discoloration. Figure 4 shows PEC # 3-3 and #3-4 after more that 40 days of exposure to a 50/50 mixture of 6 normal HCL and HNO3. The overall yellow appearance of the photo is due to the lighting when the photograph was taken.

Fig. 4. Shows The Top Surface and the
interior surface of PEC # 3-3 and #3-4 after over 40 days exposure to a 50/50
mixture to a 50/50 mixture of 6.0 normal HNO3 & HCL
PRESSURIZED WATER LEAK TEST RESULTS - AFTER ACID EXPOSURE
In order to test the cap for leaks, each was threaded onto the water column test apparatus and pressurized to a liquid head height of 26". Each cap was examined for leaks for a period of eight hours or longer. None of the caps, #3-1, #3-2, #3-3, #3-4, assembled in the third phase of testing experienced water entry. The caps #2-2 and #2-4 that proved leak tight in the second phase of testing were tested again, after 50 additional days of exposure to the acids, and neither showed signs of liquid release.
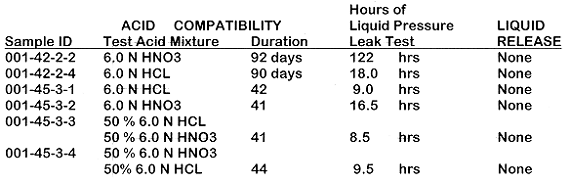
Additionally, each cap was tested for liquid release at a pressure of 7 PSI after eight months of continuous exposure to the acids. None leaked during the pressure test which lasted for 8 hours.
CONCLUSION
Nuclear Filter Technology has developed a low cost and effective means of safely venting the hydrogen gas from polyethylene bottles through a membrane that is thermally bonded to a perforated polypropylene cap. The pressure equalization cap is permeable to vapors yet impermeable to liquids. Pressure equalization caps (PEC's) allows hydrogen gas diffusion, yet retention of sub-micron particle, and liquids in the event of capsizing of the bottle. The membrane is thermally fused to the interior surface of the polypropylene caps. With the thermally bonded membrane, the pressure equalization cap is physically durable, and chemically resistant to the severe acid conditions of the liquid waste. The PEC's have undergone extensive testing including acid compatibility tests to verify vent integrity after exposure to HCL, HNO3 and mixtures of both acids for periods of 40 days and longer. At the Rocky Flats Plant, the pressure equalization caps will facilitate draining of process pipes and tanks and subsequent storage of acidic solutions that contain as much as 140 grams per liter of solubilized plutonium or uranium that pressurizes the container and generates hydrogen gas. It is planned that the PEC's will be put into use at the Rocky Flats Plant to ventilate polyethylene bottles containing solubilized plutonium in spring 1997.