ACCIDENT AT SIBERIAN CHEMICAL COMPLEX IN TOMSK-7 ON
APRIL 6,1993
V.N.Mineev, A.V.Mineev
High Energy Density Research
Center of the Institute for High
Temperatures (IVTAN) Russian Academy of
Science
Izhorskaya, 13/19, Moscow, 127412, Russia
ABSTRACT
Consideration a data from an accidental explosion of a chemical extraction
reactor with analysis of the explosion debris and area surrounding show a
realized explosive energy to 100 kg TNT explosion. Nature of an explosion is
shown to be connected to the formation of an auto-ignition explosive substance
when no mixing of the reactor contents.
The mechanism for the formation of a gas and liquid systems fuel-oxidizer is
presented. This mechanism and process has much in common with gas release events
in high-level waste tanks(Norton G. McDuffie, Westinghouse Hanford Company) and
in light water nuclear reactors, in particular in nuclear submarines.
DESCRIPTION EVENT
Accidental explosion occurred at 9:00 a.m. April 6, 1993 on plant 15 of the
Siberian Chemical Complex in Tomsk-7(1,2). Usually this radio-chemical plant
refines a fuel elements of a nuclear-power plants(3). In this case plant refines
similar but other material(4)/.Event was occurred before pause for the
time-limit work(3) when the chemical extraction reactor exploded.
The reactor had a volume of 34 m3 (5) or 34.1 m3
(6). The reactor body was made from stainless steel(6). The reactor contained 20
m3 (7) or 25 m3 (6) uranium solution/nitric acid (8)
where 310 g (6) or 500 g (7,8) Pu and 8773 kg U(6) were dissolved.
The explosive reactor was in the end technology line(9). Isotope 137 Cs was
separated before this operation(9).The uranium solution was prepared for an
extraction(6). It was into contact with an organic substance before (6). The
explosion was occurred after 6 min later than a concentrates nitric acid was
supplied in reactor(5). The cause of an explosive discharge of gas was a
chemical reaction of nitric acid with an organic substance in result a weak
motion of components (6,9,11). A concentration of the nitric acid was above the
true value (8). In addition a operator of reactor did not open the safety valve
of reactor(8). The control device of a reactor air barbotage had not been
working for quite 18 hours(8).The reactor body had working pressure 12 atm(12)
and margin of a safety equaled to 2(8). The destruction of reactor body was
occurred near 17 atm(12) or 36 atm(8). The explosion led to a partial
destruction of an industrial building(souther wall(8) and fire(6,13).
RADIOACTIVE SITUATION
The radionuclides total activity in reactor was 559.3 Ci, including
alpha-total activity - 22.4 Ci(Pu alpha activity - 19.3 Ci) and beta-activity -
536.9 Ci(6). 5% of a total activity left in environment(6). The locus of a
destructive reactor had gamma-power dose 10-15 R/h after 20 days cleansing
work(13).The explosive reactor will be covered by sarcophaqus(11,14).The maps of
a radioactive situation in a trace are shown in Fig. 1(13) and Fig.2(17). The
figures in a maps are a power doses/mR/h/ on 1 m height at April 12, 1993. The
trace was extended to the 210 degree NE from Tomsk-7(5). An original area of a
radioactive contamination was 100 km2. The wasting had time-interval
about 3 hours(3). The total radionuclides activity on the contamination
territory was valued about 40 Ci(6). A chemical analysis show existence
isotopes: 103Ru, 106Ru, 95Zr, 94Nb, 95Nb, 125Sb, 137Cs(slight)(9,11,14,16).
Content 239Pu on trace was 0,008 Ci/km2(6,14) and 0,02 Ci/km2
on plant territory(6). The trace have a same ``hot`` particles. Hot particles
has diameter several ten micrometers and radio-activity several ten 10 R(9). The
``usual`` a power dose for the industry waters in Tom` river is 500 mkR/h(15).
The trace water radioactive is about 100 km long(15).
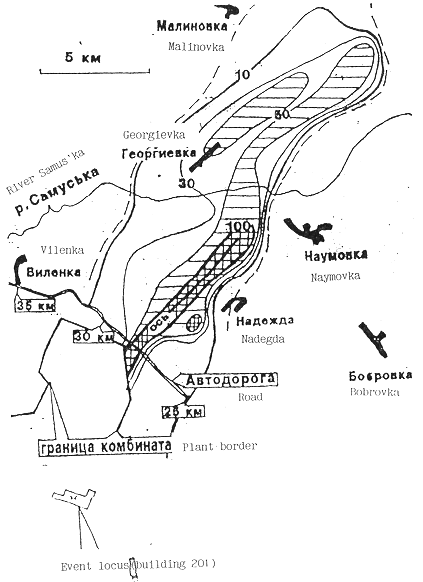
Fig. 1. The map of the radioactive
situation near Tomsk-7 on April 12, 1993. The figures are power of dose in unit
µR/h.
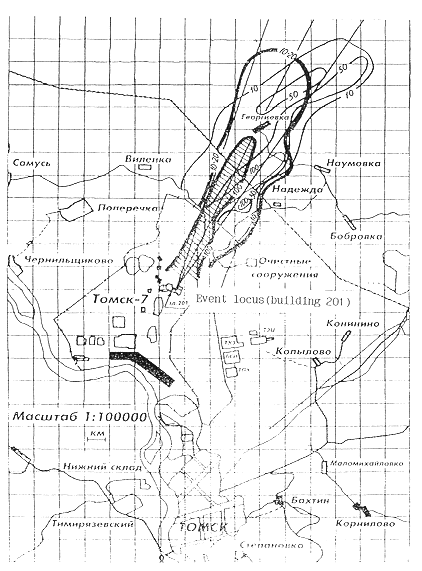
Fig. 2. The map of the radioactive
situation and plans Tomsk and Tomsk-7 on April 12, 1993. Surface measuring.
Air measuring. The figures are power of dose in unit µR/h.
RUIN ANALYSIS. TNT EQUIVALENT EXPLOSION
The explosive reactor was housed in a concrete canyon. The industrial
building scheme is shown in Fig. 3. The basic(building # 201) and the neighbor
industrial structures were partly destroyed(13). Air shock wave was generated
hence. An air shock wave was generated and propagated through the corridor in a
neighboring building and destroyed a glass-block wall(13). The view of a
destroyed basic building is shown in Fig. 4. The explosion coated the fire
roofing of the basic building area 3 m2 (6) of ruberoid(18) and
vessels with waste(industrial?)(5). The explosion destroyed a concrete floor
plate of building # 201(13). The practice show that this ruin is are the result
of 100 kg TNT internal explosion. Taking the results of a special experiment
into consideration and assuming that length of corridor was about 10 m we also
calculated the value of the resultant TNT explosion (19) at 100 kg.
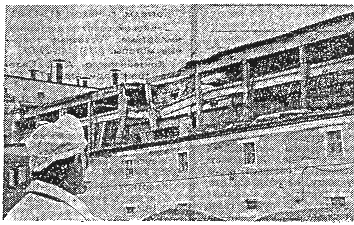
Fig. 4. The view of the destroyed
building.
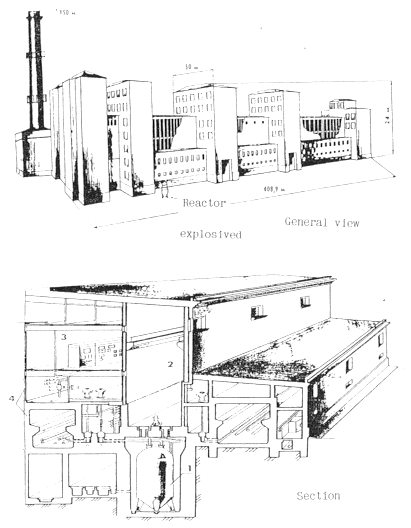
Fig. 3. The industral building 201
scheme. 1) explosive reactor, 2) cavity, 3) control unit, 4) design of
preparation and reactants supply.
EXPLOSION CHEMISTRY
According to data from Part 1 of the present paper, the explosion occurred
in one of the last stages of the process of extracting the Pu and U as shown by
the remaining isotopes(20). The organic substance was a mixture of tributil
phosphate((C4H9O)*3PO4 with TBP compound ether and kerosene(30%)), which was
usually used as the extraction agent. Pu and U were extracted from an aqueous
solution of nitric acid by means of addition F-ion(20,27). Probably the
necessity of dissolving residual U required the addition of more nitric acid
(apparently hot) into reactor(20). Explosion occurred after this operation.
Off-normal operation lies in the fact that the normal operation procedures for
the reactor were not followed. Possibly a reactor was disconnected from power.
The simultaneous disconnecting of the three devices(mixer - mechanical blade
or/and hydrodynamical generator(20), safety valve, air barbotage) lend support
to the validity of this supposition. It is noted(20) that a special step must be
used with the purpose of decreasing radiolysis in the extraction agent.
Explosive gases and liquids are produced by a combination of radiolitic and
chemical processes in the reactor. To follow N.G. McDuffie(21) let us consider
radio-chemical processes in a non-convecting extraction reactor. This products
and processes are listed in Table I. Part of the this products are fuel and
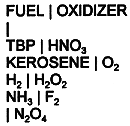
another part is oxidizer under normal conditions. This system is given in Table
II. Practically any combinations of these fuels with oxidizer are a typical
propellants(22-24). The liquid mixtures of fuels(for example, kerosene) with
nitric acid(HNO3) or N2O4 , F2 , H2
are auto-ignited after a time induction(22-26). The decomposition of mixtures: H2O2,
H2O2 + admixtures of a metals and oxides and a salts of
heavy metals, H2O2 + organic materials will produce an
explosion(22). In this case as with nuclear power plants(NPP) if ammonia is used
we shall have a simple auto-ignition of gases(propellants): NH3 + O2,
NH3 + F2, NH3 + H2O2(24).
Tri-butyl phosphate, TBP, is hydrolyzed to a mono-or di-butyl phosphate and
butyl alcohols(36).
Table I Radio-Chemical Processes Products in Extraction Reactor
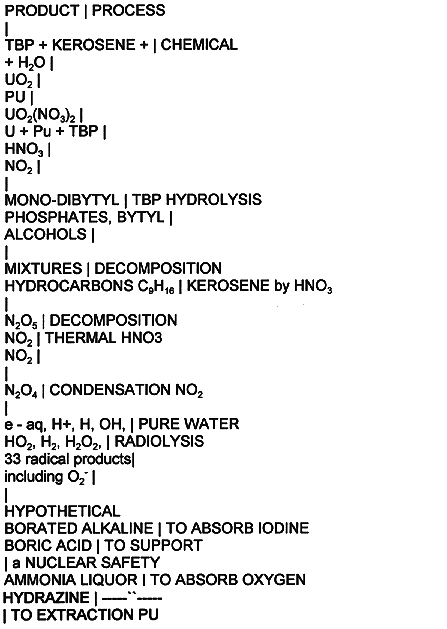
Table II Fuels and Oxidizers in Extraction Reactor
The ignition of gaseous and liquid propellants can be realized also by
cavitation of bubbles with gaseous propellants or vapor liquid propellants and
also by electrostatic discharges. For example, the flash-points are for a gas
mixtures: hydrogen(30%) - air - steam was taken place 510 C(0% H2O)
- 550 C(30% H2O), tributil phosphate - 145 C(27), butyl alcohols -
10-34 C (36) and kerosene - 28-70 C. The auto-ignition temperatures for TBP is
145 C(27), for butyl alcohols - 345-480 C(36).The liquid propellents: H2O2
+ hydrazine, H2O2 + HNO3, H2O2
+ salts of heavy metals, hydrazine + oxidizer of metals(porous) are auto-ignited
under normal conditions(37). Normally various obstacles for smooth current
promote a transition of combustion to a detonation process.
The energy of 1 kg of this propellants(liquid explosives) is about 1 kg TNT
explosive. According to (19) an explosion of 10 kg hydrogen in air is equivalent
to explosion of 100 kg TNT. That is why the TNT equivalent about 100 kg is not
unusual.
The special role of nitrogen in formation of explosive mixture was noted by
Soddy(28) and was discovered in experiments of B.S. Svetlov with colleagues(29).
It will be observed that in reality components of a broken extraction reactor
must correspond to components of high-level waste in the tank type
101-SY(Hanford). Both designs have non- convecting zones. The physical and
chemical models of flammable gas generation, retention, and release in a
high-level waste tanks was studied detail by Sinev, et al.(21).
The tank 101-SY gas release components are given in Table III. Similar
mixture of gases(% v.: H2 - 58.6, CH4 - 5.3, NH3
- 9.6, N2- 22.2, Ar - 4.3) was detonated on the chemical plant "Azot"(Rovno,
former Soviet Union) under accident 19 February 1990. Analysis of ruinis of
industrial structures under explosion of 350 m3 this mixture show
this ruinis was a result of 100 kg TNT explosion.
Table III Tank 101-SY Gas Release Components, Dry Basis
(N.G.McDuffie(21)

It has to be noted that N2O can explosive decompose in catalytic
(oxide of Ni?) reactor. This effect was used in the Germany reactive aerial bomb
type HS-296. According(21) a liquid waste has metals Na, Al, Fe, K, Ni, Zn. The
formation of a big quantity(10%) of N2, H2, NH3,
and oxides nitrogen in a liquid waste(products extraction?) and metals in an
alkali liquor can generate a hydrazoid acid and metal azides, azides of organic
and hydrazine(N2H4) or a hydrazine organic(38). Khlopin
V.G. gave an example of producing an sodium azide(starting substance for
synthesis of a hydrazoic acid and a many auto-- explosive azides) by scheme: N2H4*H2O
+ NaOH + C2H5ONO(or C5H11ONO) =
NaN3(38).
Azides and a liquid or vapor hydrozine and hydrazoid acid(NH3)
are detonators for explosives by action of thermal or a mechanical energy(heat
and mechanical sensitive explosives - initiating explosives(38)). The
interaction on falling drop of HN3 with floor lead to HN3
explosion. The fluoro-azide(FN3) is explosive at evaporation(38).
Possible local formation of sodium azide NaN3 is process with very
dangerous results. Recently work(39) showed that the ignition temperature of NaN3
was 450 C and the type of atmosphere has no effect on the decomposition.
Existence of ammonia and nitric acid or nitrous oxides in the rector can lead to
formation of the solid ammonium nitrite NH4NO2 and
ammonium nitrate NH4NO3. Mixtures of this salts are
unstable and can explode. For example this process was realized in the Turkmen
plant of a nitrogen fertilizer at June 1989. Probably high-level radioactive
particles form uranium and plutonium are pyrophoric slurries. These slurries can
ignite ruberoid roofing such a that of building # 201. Notice that a mixtures of
water with weightless particles of metals and fuel are a good explosive(it is
called ``Slurry``(30)). The fire-danger of a compounds: TBP + solvents was
discussed Flanary(27), and Benedict, Pigford(36). In general a certain
mechanisms examined could cause explosions can take place in nuclear water-water
reactors of NPP`s and nuclear submarines. The explosions of a non-working
reactors of Russian atomic submarine were reported in Feb. 1965(The Northern
Fleet)(31) and Aug.10 1985(Shkotovo-22, Pacific Fleet)(32).
IS THE TOMSK-7 EVENT THE FIRST AND LAST EXPLOSIONS IN THE
RUSSIAN ATOMIC-CHEMICAL DEVICES?
According to (6) an explosion Tomsk-7 on Apr.6,1993 was the first of that
kind. On the other hand(14) such explosions as Tomsk-7 numbered 23. It is
significant that a similar technology used in the other Siberian Chemical
Complex in Krasnojrsk-26(11).
During a Tomsk-7 explosion in 1978 the lid of a vessel was stripped loose.
In this case the building was not destroyed(8) but the lid of non-working
reactor atomic submarine was propelled to about 100 m in Shkotovo-22.
The last explosion occurred in a nuclear plant Majak at July17, 1993. The
sorbing vessel with a capacity 20 l solution 238Pu exploded (33-35).
Hence accidental explosions in an atomic plants(Sept. 29, 1957, July 1993,
Chelybinsk-40), at NPP in Chernobyl, in nuclear submarine are not unusual for
non-working(non-convecting) atomic-chemical devices.
CONCLUSION
Although the certain circumstances of an explosion Tomsk-7 on Apr.6, 1993
tally with circumstances of explosion Chernobyl on Apr.26, 1986: i.e.,- Absence
containment capable of bearing a dynamic loading,
- The control devices of a reactor had not been working,
- Operator mistake,
- Absence a technological protection from operator error,
- Other works were conducted before pause for a time-limit work,
- roof was covered with a flammable ruberoid material.
But they are not a determined physics and conditions of the event.Possibly
the process of extraction is inherently dangerous. State of immobility realize
possibility of explosion - hap-hazard reality.
RECOMMENDATIONS
This investigation shows some inability to understand a number of major
phenomena of a radio-chemical processes in the non-convecting nuclear-chemical
devices. Future work should be to concentrated on the basic research leading to
fundamental understanding:
- Radiolytic behavior of water in the presence of solutes or impurities,
- Formation kinetic of new chemical products(including explosives) or
oxidation of existing products,
- Auto-ignition of a flammable products and a combustion-detonation
transition.
- Role mixing of nuclear and a chemical active substances on generation,
retention and release of explosion.
This information is necessary for the technology safety of design basis and
off-normal accidents of extraction in processing plants for radioactive and
chemical systems.
It should not to be excluded that explosions in an extraction reactor and
explosions in light water nuclear reactor during the planned work in an active
zone have the same nature.
ACKNOWLEDGMENT
The authors are grateful to Professor Morton E. Wacks from the University of
Arizona and Doctor Norton G.McDuffie from Westinghouse Hanford Company for help
in work and support.
REFERENCES
- Russian newspaper, Apr.7, 1993
- Izvestia, Jun.9, 1993
- Izvestia, Apr.8, 1993
- Russian newspaper, Apr.8, 1993
- Komsomol`skaya pravda, Apr.9, 1993
- From the act of interdepartmental commission on the study of causes and on
the development of measures for liquidation of effect after the accident at
radio-chemical plant of siberian chemical complex in Tomsk-7. Bulletin centra
inform azii po atomnoi energii, 8`93
- Russian newspaper, Apr.10, 1993
- Moscow News, Apr.18, 1993
- Russian newspaper, Apr.14, 1993
- Izvestia, Apr.9, 1993
- Izvestia, Apr.22, 1993
- Russian newspaper, Apr.10, 1993
- Russian newspaper, Apr.20, 1993
- Izvestia, May.12, 1993
- Moscow News, May.9, 1993
- Izvestia, Apr.17, 1993
- GALISH V.F. Accident at Siberian Chemical Complex in Tomsk-7 on April 6,
1993 /6/
- Russian newspaper, Apr.29, 1993
- MARCHALL V.C. Major. Chemical Hazards. Ellis Horwood Ltd, 1987
- SINEV N.N., BATUROV B.B. Ekonomica atomnoi energetiki (in Russian), Moscow,
1984.
- NORTON G.MCDUFFIE. Flammable Gas Generation, Retention, and Release in
High-Level Waste Tanks: Physical and Chemical Models. Westinghouse Handford
Company. WHC-SA-2129-VA, WM`94. Arizona.
- VASIL`EV A.P., et al. Osnovy teorii i rascheta gitkostnuh raketnuh
dvigatelei(in Russian), Moscow, 1967
- SARNER S.F. Propellant Chemistry. Chapman & Hall, 1966
- SHTEHER M.S. Topliva i rabochie tela raketnuh dvigatelei (in Russian),
Moscow, 1976
- Sbornik by Ed. Andreeva K.K. Teoria vzruvchatuh veshestv(in Russian),
Moscow,1963
- OSIPOV S.N. Vzruvchatue veshestva i neitralizachia paro-gaso smesei (in
Russian), Kiev, 1977
- FLANARY J.R. Solvent extraction separation of uranium and plutonium from
fission products by means of tributil phosphate. Rep. 539/USA/. Geneva, 1955
- SVETLOV B.S. at el. in sborniki Gorenie i vzruv, Moscow, 1972, 1973.
- SODDY F. The Story of Atomic Energy. A new Atlantic Publ. L. 1949
- COOK M.A. The Science of Industrial Explosives. UTAH, 1974
- Izvestia, Apr.19, 1994
- Trud, Oct.25,26, 1991
- Izvestia, July 30,1993
- Izvestia, July 21,1993
- Russian newspaper, July 29,1993
- BENEDICT M., PIGFORD T.H. Nuclear Chemical Engineering. McGRAW HILL COMP.,
INC, 1957
- SCHUMB W.C., SATTERFIELD C.N., WENTWORTH R.L. Hydrogen Peroxide. Reinhold
Publ. Corp. 1955
- BAGAL L.I. Chimia i technologia iniziiruyshih vzryvchatuh veshestv (in
Russian), Moscow, 1975
- PEGG M.J., AMYOTTE P.R., LIGHTFOOD P.D., LEE M.C. Dust explosibility
characteristics of azide-based gas generants. In Proc. "Intern. Sym. on
Hazards, Prevention and Mitigation of Industrial Explosions", Bergen,
Norway, 23-26 June, 1996, pp. 7.56-7.68.






