Table I Measured Tc Losses by Volatilization

A. Jostsons, E. R. Vance, K. P. Hart, R. A. Day, B. D. Begg
and M. L. Carter
Australian Nuclear Science and Technology Organisation
(ANSTO)
PMB 1, Menai, N.S.W. 2234
Australia
ABSTRACT
The behavior of 3 wt.% each of Cs2O and Tc in Synroc has been studied as part of a strategy to develop improved methods of immobilisation of some of the HLW streams envisaged to be separated during Hanford Tank Waste remediation. Low losses of Tc (10-1-10-5%) and Cs (~ 0.1%) were measured during calcination at 750°C in argon and 3.5% H2\N2 atmospheres, with and without formic acid pre-treatment. The Tc loss by volatilisation under typical Synroc process conditions was in the range of 1-30 x 10-5%. X-ray absorption spectroscopy measurements showed that the Tc valence in the calcine varied with the calcination atmosphere. The normalized Tc differential leach rate in deionized water at 700°C, from hot-pressed specimens in which the Tc was immobilized in the metallic state, was 2.5 x 10-6 g/m2/d after 56 days. The general viability of Synroc/glass composite for immobilizing the Hanford HLW sludges is further demonstrated using an "All-blend" waste formulation containing uranium.
INTRODUCTION
Current strategies for the remediation of Hanford Tank Wastes envisage four HLW product streams: Cs, Tc, Sr/TRU and insoluble residues. The first three HLW products arise from radionuclide separations carried out on liquid wastes, including those arising from washing of sludges. One immobilization option would be to blend all HLW products prior to vitrification. About 2000 kg of Cs and 1500 kg of Tc are likely to be separated from the Hanford Tank Wastes (1). Both of these products are volatile under the oxidizing environment present in vitrification (2). The loss of Cs in vitrification depends to some degree on process parameters such as coverage of the melt by a cold cap and other melter design factors. Technetium losses above 50% have been reported by Lammertz et al. (3), and Ebert et al. (4) report Tc losses of 90% by volatilisation in laboratory tests on radionuclide doped Defense Waste Processing Facility glasses. Freude et al. (5) studied the redox behaviour of Tc in borosilicate glass melts by voltammetry and found that Tc is soluble in glass only under oxidizing conditions as Tc7+. They observed significant vaporization losses from the melt. Under reducing conditions, Tc existed in the +4 state under which the Tc solubility in the glass was negligible and TcO2 precipitates formed (5).
The use of Synroc for the immobilization of the Hanford Cs and Tc products has been discussed previously (6, 7). Jostsons et al (6) have described strategies for the immobilization of the entire Hanford tank inventory of Cs and Tc in a small Synroc plant with a capacity of 2000 kg p.a. Synroc is a dense multiphase ceramic with a high degree of aqueous durability. The phases are analogous to titanate minerals that have successfully immobilized natural radioactivity (e.g. U, Th) in a wide range of geochemical/geological settings for periods of about a billion years. Under the reducing conditions chosen for Synroc fabrication Tc is reduced to the metallic state and is alloyed with the more noble elements including Fe, present in HLW (8). The metallic alloys are microencapsulated within the titanate phases. Under less reducing conditions, Tc as Tc4+ can be incorporated in the Synroc phases rutile and perovskite (7, 9).
Here we report on a) volatility loss data for Tc and Cs in the calcination stages of Synroc processing of material containing 3 wt.% each of Cs2O and Tc in the standard Synroc precursor; b) early leach data on Synroc containing Cs and metallic Tc; and c) Tc speciation in calcination.
We also report progress on the development of a Synroc/glass composite wasteform for the sodium-rich insoluble HLW sludges at Hanford. Our strategy is to formulate a waste form that retains the extensively characterized refractory Synroc phases perovskite and zirconolite as hosts for the long-lived TRU elements, in a silicate glass matrix, and to produce it by melting at about 1400°C.
EXPERIMENTAL
Cs/Tc bearing Synroc samples were made with standard precursor containing (wt.%): Al2O3 (5.6); BaO (5.4); CaO (11.0); TiO2 (71.4); ZrO2 (6.6). 3 wt.% each of Cs2O and Tc were added as the nitrate and ammonium pertechnate respectively. Extra Fe, Ni and Cr nitrates were added to the mixture in samples where the desired form of Tc was the metal. Nitrate decomposition in Synroc precursors made by alkoxide hydrolysis is complete by about 600°C. Calcination was carried out at 750°C for one hour using flowing argon or 3.5% H2/N2 atmospheres when the Tc was targeted as Tc4+ or metallic respectively. Studies were also made to investigate the effects of formic acid denitration on the Tc volatility at about 100°C prior to calcination, using a 5:1 molar ratio of formic acid/(nitrate and pertechnate). The calcined powders were dry milled to break up agglomerates and hot-pressed in collapsible metal bellows at 1250°C/20MPa. The redox conditions under hot-pressing were generally controlled by the addition of 10 wt.% of Ti metal powder or 10 wt.% NiO powder to the calcine before hot-pressing the samples in which the desired state of Tc was metallic or tetravalent respectively.
Samples were characterized by x-ray diffraction, scanning electron microscopy and analytical transmission electron microscopy. Leaching measurements were made at 70°C by a modified MCC-1 method on polished discs, 1 cm in diameter and 0.2 cm thick. Standard 7-day PCT tests were also carried out as described elsewhere (7).
X-ray absorption spectroscopy on the Tc speciation was carried out on finely powdered samples of material corresponding to the drying and calcination stages of the hot-pressing process route. The measurements themselves were carried out on the radioactive line 4-2 at the Stanford Synchrotron Radiation Laboratory. Valence standards for this work were dried NH4TcO4 bearing materials, together with TcO2 and Tc formed by heating NH4TcO4 in argon or 3.5%H2/N2 respectively at 900°C. The Tc speciation in these latter two standards was confirmed by XRD.
Volatile losses on calcination were studied by placing ~2g (oxide equivalent) of the dried material, with or without formic acid pre-treatment, in an alumina boat, placing the boat inside a ~60 cm long open ended silica tube, and then inserting the assembly in a gas-tight alumina tube contained in a furnace, such that the silica tube projected at least 30 cm beyond the end of the furnace. The chosen calcination gas flowed through the experimental chamber. A water bubbler was also placed on the downstream side of the furnace. After calcination of the sample for one hour at 750°C the silica tube was closely inspected for deposited material along its length. The tube was then subjected to alkali (1M NaOH solution) and acid (2M HNO3 solution) washes, and the washings were analyzed for Tc and Cs by liquid scintillation counting and atomic absorption spectroscopy respectively. The water in the bubbler was similarly analyzed.
Melting of the "All blend" plus additives mixtures in alumina crucibles was carried out at the 50 gm scale at temperatures of ~1350°C, using flowing atmospheres of argon or air. The melts were cooled at 5°C/minute to about 400°C and thereafter more slowly to ambient temperatures.
RESULTS AND DISCUSSION
Tc and Cs Volatility
The results of the measured Tc volatilisation losses, with and without formic acid pre- treatment, are shown in Table I. The results represent total losses of volatiles deduced from the combined condensed materials removed by washing of the silica tube and those trapped in the water bubbler. When formic acid pre-treatment was used, only a few drops of condensed steam, and no more than ~1 µg of solid material deposited on the inside of the silica tube. A clearly observable deposit of reddish-brown colored material was deposited on the cold parts of the tube from a sample calcined in argon but not pre-treated with formic acid.
The measured Cs loss was ~ 0.1% in every case, a value in good agreement with previous determinations on Synroc-C (8).
Table I Measured Tc Losses by Volatilization

The results shown in Table I indicate that volatilisation of Tc is effectively suppressed by both argon and the reducing atmosphere (3.5%H2/N2) during calcination. The use of the formic acid pre-treatment results in significant Tc volatility reductions in neutral atmosphere calcination and are broadly consistent with the observations of Ito and Kanno (10) on simulated HLLW containing noble metals.
X-Ray Absorption Spectroscopy of Tc Speciation During Processing
Figure 1 shows the near edge behaviour of the Tc K absorption edge at 21.064 keV in calcined materials and those of the Tc valence standards. Calcination in argon yielded an average Tc valence state between +4 and +7, whereas calcination in 3.5%H2/N2 gave an average valence state between 0 and +4. These observations confirm the expected decrease in volatility of Tc with decreasing valence state.
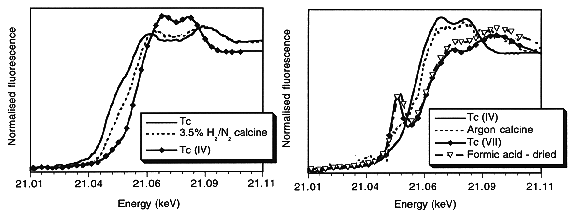
Fig. 1. XANES spectra from Tc-bearing
preparations after drying and calcination stages of Synroc production, together
with spectra from Tc metal, TcO2 (Tc(IV)) and NH4TcO4
(Tc (VII)) standards.
The effect of the formic acid pre-treatment requires further comment. The x-ray absorption spectrum of a formic acid treated specimen, dried in air at about 100°C, and not calcined, suggests that the formic acid treatment in our case had not reduced the Tc valence from +7. This implies that the role of the formic acid treatment is via chemical denitration which results in the reduction of oxidizing NOx products during calcination, as noted by others (3, 10). Hence, there will be less tendency for the Tc to remain in the volatile Tc+7 state in the argon calcination atmosphere than if NOx were present.
Chemical Durability
In Synroc-C, Tc is immobilized in alloys containing noble metals from fission products in HLLW together with process contaminants such as Fe, Ni etc. (8). In the present study we targeted an alloy of Fe, Ni, Cr for Tc as deliberate noble metal addition is not realistic for separated waste streams of Tc. The leaching data shown in Table II are for specimens calcined in 3.5%H2/N2 without formic acid pre-treatment. The calcine was hot-pressed in a graphite die without addition of Ti powder. Under these process conditions, the Tc was metallic and alloyed with Fe and Ni. The Cr was found to be within the ceramic phases. Other studies have shown that hot-pressing with Ti metal additions would have reduced Cr+3 to the metallic state.
Table II Normalized Tc Differential Leach Rates from Synroc
Containing 3 wt.% Tc in Distilled Water at 70°C

The leach rates are equivalent to total release of Tc from Synroc as species deposited on the leach vessel walls measured after acid stripping contributed < 1% to totals. These leach rates are equivalent to those from Synroc-C where the Tc is microalloyed with noble metals (7).
Synroc/Glass Composites for Immobilization of Hanford HLW sludges
We have previously demonstrated (6, 11) that high waste loadings are achievable in a Synroc/glass composite melted at 1350-1400°C using rare earth simulants for uranium and actinides with good leach resistance as measured in 7-day PCT tests. This has involved the addition of TiO2, SiO2, Al2O3 and CaO to the simulated sludge. More recently we have employed an active "All blend" HLW sludge simulation (12) which contains 14.3 wt.% of U3O8. In melts containing 60% dry "all blend" sludge and 40% of additives, most of the uranium is partitioned to zirconolite (in air) or the related pyrochlore (in argon). However, some (Na, Ca) phosphate is formed and (Na, Ca)2 U2O7 is present when melted in air. Both of these phases are relatively soluble in water. By increasing the addition of Al2O3 more sodium is converted to nepheline which has acceptable leach resistance in water. A reducing or argon environment during melting will be necessary to ensure that soluble U6+ phases are eliminated totally.
CONCLUSIONS
The present study has shown that Tc volatility under the reducing conditions of Synroc processing is negligible and linked to the stability of metallic Tc or low valence species. Volatility is not important in the closed bellows hot-pressing of calcined material. The very low release rates of Tc from Synroc in aqueous environments, as measured by the MCC-1 tests, is consistent with the observations on the geochemical behavior of metallic Tc in the Oklo natural fission reactors (13).
Technetium-99 and 135Cs, because of their long half-lives and mobility in the environment, contribute significantly to the long-term risk associated with radioactive waste disposal. The low release rates of these materials from Synroc in aqueous media, coupled with their low volatility in Synroc processing, provide incentives to condition these HLW streams separately from the Hanford sludges in order to avoid difficulties with the maintenance and operation of the off-gas systems in vitrification.
The viability of a Synroc/glass composite wasteform for the immobilization of HLW sludges from Hanford tanks at high waste loadings is confirmed. Our work with uranium-rich sludges indicates that a reducing or argon environment during melting is likely to be essential to avoid the formation of U+6 containing phases. This requirement was not immediately obvious from earlier work using non-radioactive simulants.
A Synroc/glass composite wasteform could be produced with cold-crucible melters at rates adequate for Hanford remediation. The Synroc/glass composite could be a useful alternative to vitrification if high waste loadings cannot be achieved with borosilicate glass.
ACKNOWLEDGMENTS
We thank S. Conradson for assistance with the x-ray absorption measurements which were conducted at the Stanford Synchrotron Radiation Laboratory, supported by DOE and NIH.
REFERENCES