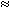 106 years) and require a durable
immobilization system.
106 years) and require a durable
immobilization system.
D. Singh, A. S. Wagh, M. Tlustochowicz, and V. Mandalika
Energy Technology Division
Argonne National Laboratory
Argonne, IL
60439
ABSTRACT
Over the last few years, Argonne National Laboratory has been developing room- temperature-setting chemically bonded phosphate ceramics (CBPCs) for use in solidifying and stabilizing low-level mixed wastes. The focus of this work is development of CBPCs for use with fission-product wastes generated from high-level waste (HLW) tank cleaning or other decontamination and decommissioning activities. The volatile fission products such as Tc, Cs, and Sr removed from HLW need to be disposed of in a low-temperature immobilization system. Specifically, this paper reports on the solidification and stabilization of separated 99Tc from Los Alamos National Laboratory's complexation-elution process. Using rhenium as a surrogate form technetium, we fabricated CBPC waste forms by acid-base reactions. Dense and hard ceramic waste forms are produced in this process. The principal advantage of this technology is that the contaminants are immobilized by both chemical stabilization and subsequent microencapsulation of the reaction products. This paper reports the results of durability studies conducted on waste forms made with 35 wt.% waste loading. Standard leaching tests such as ANS 16.1 and PCT were conducted on the final waste forms. In addition, stability of the waste forms in aqueous environments was evaluated by long-term water-immersion tests.
INTRODUCTION
The current volume of high-level waste (HLW) across the Department of Energy
(DOE) complex is several hundred thousand cubic meters. The presence of fission
products such as 99Tc in the HLW poses myriad problems; this
radionuclide, for example, is highly volatile and may escape into offgas during
HLW vitrification, thus generating a secondary waste stream that requires
additional stabilization/solidification (S/S). Technetium is readily oxidized to
a highly soluble pertechnetate form that is extremely difficult to immobilize.
Technetium and cesium (135Cs) have extremely long half-lives (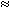 106 years) and require a durable
immobilization system.
106 years) and require a durable
immobilization system.
The approach that is being considered is to remove fission products from HLW and dispose of them separately. Removing fission products from the HLW reduces the overall waste volume and radioactivity levels of HLW, thus simplifying waste-handling operations. In this regard, several separation technologies are being developed as part of DOE's Efficient Separations and Processing Crosscutting Program. These separation technologies, along with other decontamination activities, result in waste streams that are rich in fission products and, if not returned to the bulk HLW stream, require S/S for safe disposal in compliance with federal regulations. Currently, no robust and reliable low-temperature technologies are available to immobilize these fission-product wastes. A low-temperature stabilization and immobilization technology could produce durable waste forms for long-term storage and disposal of the fission products and/or the "loaded" separating agents used to remove fission products from the HLW streams. To this end, we are developing and demonstrating a low-temperature treatment and stabilization technology based on chemically bonded phosphate ceramics (CBPC).
CBPCs are dense and hard materials that can be processed at low temperatures (1,2). Phosphates exhibit high solid-solution capacity for incorporating heavy metals, actinides, and rare-earth contaminants (3). Also, the very low solubilities of phosphates of heavy metals, actinides, and rare earths (4) indicate that phosphate-bonded ceramics should be effective in stabilizing these contaminants. In addition, the durable natural-analog monazite and apatite minerals (5) suggest that phosphates are good hosts for radionuclides. The present developmental program on phosphate waste forms is being conducted to utilize their attractive properties in S/S of fission products, especially 99Tc, because fabrication can be achieved at room temperature or slightly elevated temperatures to minimize off-gassing.
The goal of this work is to incorporate simulated wastes of partitioned 99Tc generated by the Los Alamos National Laboratory (LANL) complexation-elution process in magnesium-phosphate-based ceramic systems, and to conduct assessments of the resulting waste forms to establish their performance. In addition, physical and microstructural characteristics of the final waste forms are being determined to gain insight into the stabilization mechanisms of the phosphate ceramics and the durability of the final waste forms. Results to date show that phosphate ceramics are a viable S/S technology for treating 99Tc-containing waste streams.
FABRICATION OF CBPC WASTE FORMS
Phosphate-bonded ceramics can be classified as acid-base cements because
they are fabricated by chemical reactions between an oxide powder and an acid
solution. A magnesium phosphate ceramic was prepared by reacting calcined
magnesium oxide powder with an acid solution (6). Particle size of the starting
magnesium oxide powder was 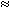 8 µm. The powder
was continuously added to the acid solution, and the mixture was vigorously
mixed to a desired consistency and then transferred to a mold and allowed to
set. The mix hardened in
8 µm. The powder
was continuously added to the acid solution, and the mixture was vigorously
mixed to a desired consistency and then transferred to a mold and allowed to
set. The mix hardened in 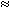 2 h to form hard and
dense monoliths. After complete curing for >7 days, the monoliths were
removed from the molds for physical and chemical evaluation.
2 h to form hard and
dense monoliths. After complete curing for >7 days, the monoliths were
removed from the molds for physical and chemical evaluation.
Specific CBPCs are being developed for S/S of 99Tc partitioned
from HLW by LANL's sorption process. Surrogate formulations of the separated Tc
waste stream based on LANL's complexation-elution process have been prepared and
the waste subsequently incorporated in the phosphate ceramic. However, in this
work, Re was used as a surrogate for Tc. The composition of the waste stream
used was 1 M NaOH, 1 M ethylenediamine, and 0.005 M Sn(II) and 0.00005 M Re (7).
Loading of the waste stream in the final waste form was
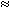 35-40 wt.%. The final waste forms were
fabricated by mixing the liquid waste with the acid solution in desired weight
percentages. Rhenium was added so that in the final waste forms its content was
3 ppm. In addition, CBPC waste forms were fabricated in which Re was directly
added as ReO2.2H2O. The net Re content in these waste
forms was maintained at either 5 or 50 ppm, which is the expected range of
99Tc in the actual waste. Subsequently, the powders were added at a
slow rate and thoroughly mixed. The slurry was then poured into a mold and
allowed to set. After complete setting, all of the specimens formed hard
ceramics that were used for further evaluation.
35-40 wt.%. The final waste forms were
fabricated by mixing the liquid waste with the acid solution in desired weight
percentages. Rhenium was added so that in the final waste forms its content was
3 ppm. In addition, CBPC waste forms were fabricated in which Re was directly
added as ReO2.2H2O. The net Re content in these waste
forms was maintained at either 5 or 50 ppm, which is the expected range of
99Tc in the actual waste. Subsequently, the powders were added at a
slow rate and thoroughly mixed. The slurry was then poured into a mold and
allowed to set. After complete setting, all of the specimens formed hard
ceramics that were used for further evaluation.
RESULTS AND DISCUSSION
Physical Properties
Density of the magnesium phosphate-LANL waste forms with 35 wt.% loading was 1.8 g/cm3. The corresponding porosity was 4%. Such low porosity values are highly desirable for minimizing water intrusion in the final waste form.
Phase Analysis
Figure 1 shows an X-ray diffraction (XRD) pattern of the CBPC-36 wt.% eluted waste form. The major phase is of magnesium phosphate system, a highly insoluble phase. In addition, there is some unreacted magnesium oxide. Using XRD on the final waste forms, we determined that both crystalline and noncrystalline phases of magnesium phosphate are present as the binding phases. Analysis of noncrystalline phases, which is much more complex and requires a combination of techniques, is currently the focus of our work.
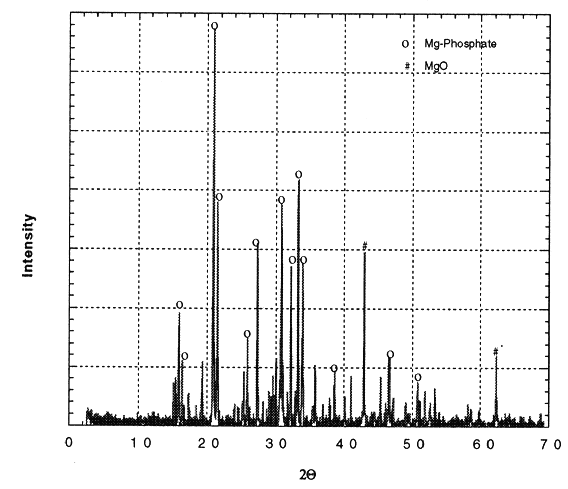
Fig. 1. XRD Pattern of CBPC-35 wt.%
eluted waste form.
Microstrucrural Analysis
Figure 2 is a photomicrograph of the fracture surface of CBPC-35 wt.% eluted waste form. As seen in this figure, the fabricated ceramic is extremely dense. Needlelike crystals of the magnesium phosphate phase are evident. Scanning electron microscopy (SEM) was also used to map the distribution of the metal contaminants in the magnesium phosphate final waste form. Rhenium was found to be well distributed and physically microencapsulated by the phosphate matrix, and we believe that this phenomenon, along with chemical stabilization, leads to excellent performance of the phosphate systems in preventing leaching of the Re during the tests discussed below. It is expected that Tc will behave similarly.
Compression Strength
Compression strength of fully cured waste form samples was measured with an Instron machine on samples of 0.5 in. diameter and 1-2 in. length. For monolithic magnesium phosphate specimens, the average compression strength was 4320±954 psi. This value is higher than those of other room-temperature-setting materials such as portland cement.
ANS 16.1 Test
Leaching studies on the fabricated waste forms were conducted according to the standard American Nuclear Society's ANS 16.1 test and the Product Consistency Test (PCT) (8,9). The ANS 16.1 test was followed to evaluate the diffusion constants and the leachability indexes for Re; results are presented in Table I. As expected, diffusivity of the contaminants is extremely low, indicating excellent stabilization of Re in the phosphate matrix system. The determined leachability indexes range from 10 for 3 ppm loading to as high as 12 for 50 ppm Re loading, significantly higher than Nuclear Regulatory Commission's passing criterion of 6. (A higher leachability index indicates better retention of the contaminant in the final waste form.) These results are further evidence of the superior containment characteristics of the CBPC final waste forms. The reason for this superior immobilization is chemical stabilization of the contaminants in the matrix due to reaction between the contaminant and the acid solution, followed by physical encapsulation within the dense phosphate matrix. Also, physical encapsulation immobilizes the contaminants in the matrix, thus forming an excellent final waste form.
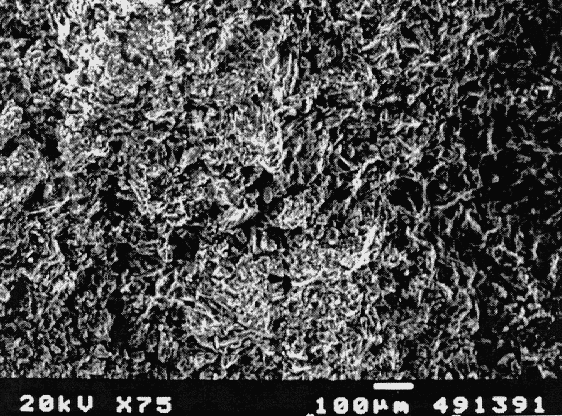
Fig. 2. SEM Photomicrograph of
fracture surface of CBPC-35wt.% eluted re final waste form.
Table I. Diffusion Constants and Leachability Indices for Re in
CBPC-Eluted Re Waste Forms
Product Consistency Test
Product Consistency Test (PCT) was conducted on CBPC-bonded 35 wt.% LANL
eluted waste, and also on CBPC waste forms in which Re was directly added as ReO2.2H2O
to concentrations of 5 and 50 ppm of Re in the final waste form. PCT tests were
carried out at 90°C for 7 days in Teflon containers holding 1 g of 100-200
mesh waste form particles in 10 mL of deionized water. Table II summarizes the
leaching rates observed for each type of waste form sample; leaching of Re is
extremely low for all three samples. These values are significantly lower than
those reported for 99Tc release from borosilicate glass (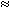 0.12 g/m2) (10). Moreover, no significant
change was observed in the leaching rate of Re with an increase in concentration
of Re in the waste form. It is clear that Re cannot act completely as a
surrogate for 99Tc, but based on redox chemistry, it is more prone
to becoming oxidized to the perrhennate form that is 99Tc to the
pertechnetate form. Hence, Re is more susceptible to leaching out than 99Tc.
0.12 g/m2) (10). Moreover, no significant
change was observed in the leaching rate of Re with an increase in concentration
of Re in the waste form. It is clear that Re cannot act completely as a
surrogate for 99Tc, but based on redox chemistry, it is more prone
to becoming oxidized to the perrhennate form that is 99Tc to the
pertechnetate form. Hence, Re is more susceptible to leaching out than 99Tc.
Table II. Results of PCT on Phosphate-Bonded Waste Forms
Long-term Water Immersion Study
To study the long-term durability of the final waste forms in an aqueous
environment, water immersion studies were initiated. As done for the ANS 16.1
standard, samples were immersed in deionized water (pH
 5.5). Samples were removed at regular
intervals, dried, and weighed to observe any weight change. Results for CBPC-36
wt.% eluted waste forms are shown in Fig. 3. Initially, an increase in weight
was observed. Thereafter, the weight of the sample decreased and held constant.
It is not clear why there was an initial weight increase and then a subsequent
decrease, but the cause was probably interaction of unreacted MgO and water.
After 90 days, only a small decrease (
5.5). Samples were removed at regular
intervals, dried, and weighed to observe any weight change. Results for CBPC-36
wt.% eluted waste forms are shown in Fig. 3. Initially, an increase in weight
was observed. Thereafter, the weight of the sample decreased and held constant.
It is not clear why there was an initial weight increase and then a subsequent
decrease, but the cause was probably interaction of unreacted MgO and water.
After 90 days, only a small decrease ( 0.5 wt.%)
was seen in the weight of the specimens immersed in the deionized water.
0.5 wt.%)
was seen in the weight of the specimens immersed in the deionized water.
Fig. 3. Variation in weight of CBPC-35 wt.% LANL eluted final waste forms
exposed to deionized water.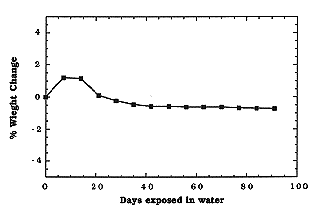
Compression Strength Before and After Water Immersion Test
Figure 4 compares the average compression strength of the CBPC-35 wt.%
eluted waste forms before and after long-term (90-day) water immersion tests.
Specimen strength after exposure to deionized water were 1700 ± 372 psi.
These values are lower than the average strength of the waste form before the
long-term durability test ( 4320 psi). The
resulting strength of the waste forms is exceptionally good and satisfies the
regulatory requirements (>500 psi) after a 90day exposure to an aqueous
environment. The results given here for weight change and compression strength
clearly indicate that CBPCs will be durable waste forms.
4320 psi). The
resulting strength of the waste forms is exceptionally good and satisfies the
regulatory requirements (>500 psi) after a 90day exposure to an aqueous
environment. The results given here for weight change and compression strength
clearly indicate that CBPCs will be durable waste forms.
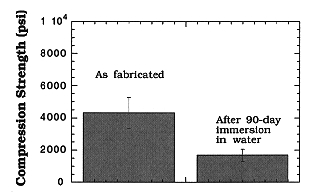
Fig. 4. Compression strengths of
CPBC-35 wt.% eluted waste forms in as-fabricated state and after exposure to
aqueous environment.
SUMMARY
This paper reports on the durability of magnesium-phosphate-bonded ceramics
for stabilization of eluted Tc wastes from sorption process at low temperatures.
Physical and chemical characterization of phosphate materials has shown them to
be physically, chemically, and mineralogically stable, with properties superior
to those of other room-temperature-setting cements. Surrogate formulations of Tc
eluted waste stream have been incorporated and stabilized in CBPCs at loadings
of  35 wt.%. Long-term durability studies using
the ANS 16.1 standard test)showed excellent containment of the radioactive
surrogates (Re for Tc) in the phosphate matrix when exposed to deionized water.
Excellent retainment of contaminants in the CBPC matrix was further confirmed by
the PCT test. In addition, CBPC-based final waste forms showed no significant
weight changes after exposure to aqueous media for
35 wt.%. Long-term durability studies using
the ANS 16.1 standard test)showed excellent containment of the radioactive
surrogates (Re for Tc) in the phosphate matrix when exposed to deionized water.
Excellent retainment of contaminants in the CBPC matrix was further confirmed by
the PCT test. In addition, CBPC-based final waste forms showed no significant
weight changes after exposure to aqueous media for
 90 days, indicating the highly insoluble nature
of the phosphate matrix.
90 days, indicating the highly insoluble nature
of the phosphate matrix.
ACKNOWLEDGMENT
This work has been supported by the U.S. Department of Energy, Office of Technology Development (EM-50), as part of the Efficient Separations and Processing Crosscutting Program, under Contract W-31-109-Eng-38.
REFERENCES
*Work supported by the U.S. Department of Energy, Office of Technology Development (EM-50), as part of the Efficient Separations and Processing Crosscutting Program, under Contract W-31-109-Eng-38.