Vyacheslav M. Abashkin, Anna K. Nardova, Igor V. Mamakin,
Gennadiy F. Egorov, and Evgeniy A. Filippov
Russian Research Institute of
Chemical Technology - 33 Kashirskoe Ave. Moscow 115230, RUSSIA
Tel.
(095)324-6035; FAX: (095) 324-7906; E-mail: vm@abas.msk.ru
ABSTRACT
Crown ether Dibenzo-21-crown-7 (DB-7) has demonstrated a high capability for removing cesium-137 from nitric acid solution (1). However there is a very important problem concerning changes in physical and chemical properties of such mixtures as a result of high irradiation. High radiation doses may cause a decrease in extraction efficiency and selectivity, formation of hazardous radiolysis products, disturbance hydrodynamics, and even decay of extractant. It is necessary to estimate such changes before extraction testing on "hot" solutions.
A special procedure, earlier developed in our Institute, was used for testing of radiation and chemical stability of extraction mixtures. This method is based upon continuous control of extraction parameters - distribution coefficients of specified radionuclides, their hold-up in organic phase, of hydrodynamic properties - kinetics of separation of mixed phases, surface tension, etc., and formation of radiolysis products.
The dose rate, determined using a ferrosulfate dosimeter was 1.7 W*h/l (1.05*1016 eV/ml*s) taking into account the electronic density of organic solution. Maximum total dose of gamma-irradiation was 80 W*h/l (1.6*1021 eV/ml). The specimens of irradiated organic solution - DB-7 in the mixture of fluorinated alcohol (FA) and higher alcohol - after phase separation was analyzed to determine the hydrodynamic parameters. Both organic and aqueous phases were analyzed to estimate the decay of extractant and the composition of radiolysis products.
The primary emphasis for the first radiolytic experiments was given to the estimation of the FA decay by means of measuring the fluoride-ion concentration in the aqueous phase. It was shown that this value increases through the dose of 60 W*h/l and then decreases again Such behavior may be attributed to an interaction of the fluoride ion with nitric acid and formation of volatile or organic phase extractable compounds. Our tests performed on real HLW acid solutions in P/A "Mayak" also demonstrated that solutions of Dibenzo-21-crown-7 in some fluorinated alcohols have a high resistance to radiolysis.
INTRODUCTION
Crown ethers - stereospecific macrocyclic complexones - exhibited high selectivity for certain cations and demonstrated good applicability for decontamination of high-level waste (HLW) from long-lived radionuclides: Strontium-90 and Cesium-137. Dibenzo-18-crown-6 (DB-7) and its derivatives are the most promising agents in a solvent extraction of Cs from acidic solutions (1).
As a rule, the extractant used for radwaste decontamination is exposed to as much as 50 kGy per extraction-stripping cycle depending on the extraction regime and equipment. For a cyclic scheme, the total radiation doses may reach tens of MGy (2). Usual consequences of radiolysis are: degradation of the extractant, drop in the recovery efficiency, formation of destruction products, reduction of the selectivity, and bad phase separation.
Unfortunately, the limited availability of crown ethers and especially a lack of experimental data for reprocessing of highly irradiated and concentrated aqueous solutions using crown ethers as extractants have somewhat inhibited the evaluation and recognition of crown ethers as new agents in the reprocessing of industrial nuclear fuel and radioactive waste. Nevertheless, the possible application of crown ethers for recovery of radionuclides [Sr, Cs, transuranium elements (TRU)] from real radioactive waste solutions has been reported (3,4).
The following parameters of the extraction mixture were controlled under irradiation: concentration of DB-7 in organic phase; some hydrodynamic properties of the extracting mixture - surface tension, phase demixing rate ; and accumulation of radiolysis products.
EXPERIMENTAL
In compliance with this technique we irradiated aqueous/organic (1/2 vol.) mixture. The aqueous solution was 3M HNO3, and organic phase was 0.1 M solution of DB-7 in the mixture of fluorinated alcohol (FA) and isododecyl alcohol (IDA) - 3:2. This two-phase system was gamma-irradiated by means of 60Co in special stainless steel thermostat provided with continuous shaking (rotation velocity of the shaker was 2500 turns per minute) and intensive bubbling of gaseous oxygen (30-40 ml/min.).
The dose rate, determined by means of ferrosulfate dosimeter, was 1.7x103 W/l (1.7 Gy/s ) taking into account the electronic density of organic solution. Maximum total dose of gamma-irradiation was 80 W*h/l (2.88 Gy). The specimens of irradiated organic solution after phase separation were analyzed to determine residual concentration of DB-7 and hydrodynamic parameters. Both organic and aqueous phase were analyzed to estimate the decay of extractant and composition of radiolysis products, using UV-spectrometry.
RESULTS
Hydrodynamic Effects
The following hydrodynamic characteristics of irradiated DB-7 solutions were controlled: interfacial tension (IT), and a rate of demixing (RD). The IT was determined by a stalagmometer using capillary technique. Aqueous nitric acid solutions were used as dispersive medium and the extractant as dispersed phase. The hydrophilic surface capillarity prevents a spread of an organic drop on the end of glass capillary. The IT was calculated as:
where "v" - volume of "n" drops;
![]() r
-density difference of organic and aqueous phase; "g" - acceleration
of gravity; and "r" - capillary radius. The demixing rate was measured
in special glass mixer with inner diameter of 2.5 cm. The volume of every phase
was 10 ml; time of intermixing - 2 minutes.
r
-density difference of organic and aqueous phase; "g" - acceleration
of gravity; and "r" - capillary radius. The demixing rate was measured
in special glass mixer with inner diameter of 2.5 cm. The volume of every phase
was 10 ml; time of intermixing - 2 minutes.
The effect of irradiation on RD for different aqueous solutions is shown on Fig. 2. RD is practically unchangeable when the irradiated extractant contacts concentrated (3 M) nitric acid solution. Perhaps the acidic radiolysis adducts promote low interfacial activity. Meanwhile, with dilution of the HNO3 solution, RD decreases evidently, and there is sharp drop of RD just for the first step of gamma-irradiation (up to 20 W*h/l). For comparison the same parameter of dicyclohexano-18-crown-6 (DCH-6) is indicated.
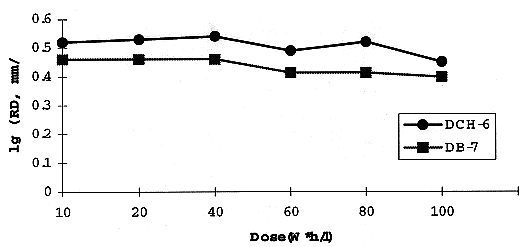
Fig. 1 Effect of irradiation on a
rate of phase demixing (RD).
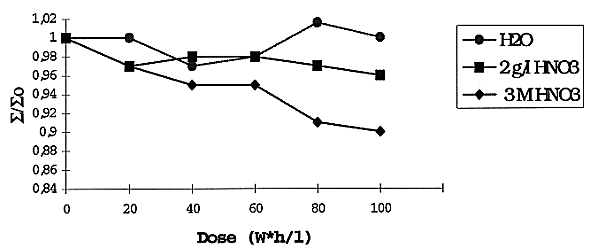
Fig. 2. Effect of irradiation on
interfacial tension (IT) of the extractant: 0.1 M DB-7 + 60% FA + 40% IDA.
A comparison of IT as a function of irradiation is given in Fig. 3. There is practically the same IT value for all types of aqueous solutions and relatively low effect of the total dose, except of the more concentrated nitric acid solution. A lack of simple correlation between RD and IT may be caused by different chemical and hydrodynamic factors discussed earlier (2).
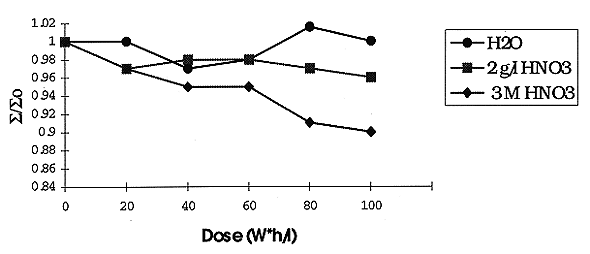
Fig. 3. Effect of irradiation on
interfacial tension of extractant: 0.1 M DB-7 + 60% FA + 40% IDA
Accumulation and Composition of Radiolysis Products
The concentration of DB-7 in organic solution on irradiation was measured spectrophotometrically using technique described in (6). Analysis of Infrared Spectra of irradiated extractant in the range since 1500 thru 2000 cm-1, has shown a formation of carbonyl derivatives (1730 cm-1), organic nitrates (1640 cm-1) and nitrites (1680 cm-1) produced in result of oxidizing and nitration of the diluent - higher alcohol. Radiation yields for these adducts - measured using appropriate kinetic diagrams (Fig. 4). are: 10.5 (R=CO), 1.3 (R-ONO2), and 1.5 (R=NO2) molecules per 100 eV.
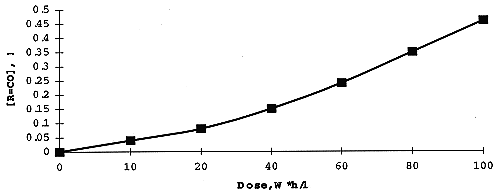
Fig. 4. Formation of carbonyl adducts
while irradiation of the extractant
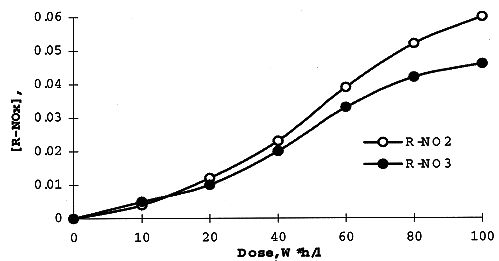
Fig. 5. Formation of nitrate and
nitrite adducts while irradiation of the extractant
Special attention was paid to an accumulation of fluoride-ions in the aqueous phase as a result of radiation decay of the solvent (FA) given in Fig. 6. The concentration of F- increases up to a total dose 60 W*h/l and then slightly decreases. A possible reason for the maximum is the interaction of fluoride-ions with nitric acid and a formation of volatile or extractable compounds. Initial radiation yield of F- is 5.5 ions per 100 eV, while the highest concentration of fluoride is 0.12 g*ion per liter - below the permissible corrosion-proof level of the standard extractor.
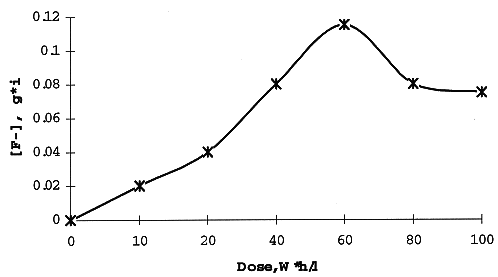
Fig. 6. Accumulations of
fluoride-ions in aqueous phase as a result of gamma-irradiation
Concentration of DB-7 on Irradiation
During gamma-irradiation the decay of DB- 7 and its concentration in organic phase was constantly measured. It was shown (Fig. 1) that less than 10% of initial crown ether was lost on irradiation up to 100 W*h/l. It is additional evidence of surprisingly great radiation stability of macrocyclic polyethers (5).
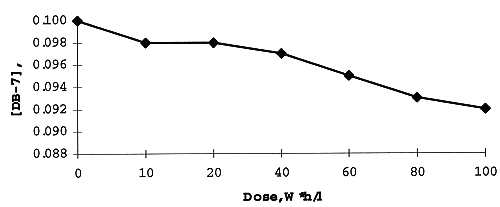
Fig. 7. Concentration of DB-7 during
gamma-irradiation.
CONCLUSIONS
The subject extractant - solution of DB-7 in the mixture of higher and fluorinated alcohol - demonstrated a high chemical stability during gamma-irradiation in two-phase system with nitric acid solution. The distribution coefficient of 137Cs and its separation factors from other radionuclides, have high and stable values over the irradiation range (at least up to 80 W*h/l).
The irradiation causes a high yield of carbonyl adducts due to the oxidation of higher alcohol and fluoride-ion from the decay of fluorinated alcohol. There is no loss of crown ether after irradiation.
Thus, DB-7 in the mixed solvent (FA+IDA) may be recommended in the reprocessing of HLW for decontamination of liquid waste from 137Cs .
REFERENCES