Table I Average Compositions of Zr Calcine and Sodium Bearing Waste
T. A. Todd, K. N. Brewer, J. D. Law, D. J. Wood, T. G. Garn,
R. D. Tillotson, P. A. Tullock, and E. L. Wade
Lockheed Martin Idaho
Technologies Co.
ABSTRACT
Separation technologies for the treatment of radioactive wastes, including highly radioactive liquid wastes, solid calcined wastes, mixed wastes and contaminated groundwater are under development at the INEL. An overview of the technologies under development, specific applications of the technologies, and the benefits derived from use of the technologies are discussed. The INEL has recommended radionuclide partitioning technologies for the treatment of high activity wastes. This alternative involves the separation of radionuclides and possibly RCRA metals from the existing liquid tank waste and HLW calcine. The results of recent tests, including countercurrent pilot plant demonstrations of solvent extraction processes with actual tank waste, are presented. Removal of radionuclides to levels below the NRC Class A LLW criteria has been demonstrated. Results of tests to remove cesium and strontium from contaminated groundwater using highly-selective ion-exchange sorbents are also presented. Removal of cesium and strontium to below drinking water standards (119 pCi/L and 8 pCi/L has been demonstrated.
INTRODUCTION
The Idaho Chemical Processing Plant (ICPP), which is located on the Idaho National Engineering Laboratory (INEL), reprocessed irradiated nuclear fuel from 1953 to 1992 to recover uranium-235 and krypton-85 for the U.S. Department of Energy. The resulting acidic liquid radioactive waste was solidified to a high-level waste (HLW) calcine, and stored in stainless-steel bins enclosed in concrete vaults. About 3800 m3 of radioactive HLW calcine is currently stored at the ICPP. In addition to the HLW calcine, approximately 6.9 million liters of high-activity acidic-liquid waste are stored at the ICPP. This liquid is a result of decontamination activities, evaporator bottoms and solvent wash activities and cannot be calcined directly because of its high sodium content.
The HLW calcine is composed primarily of metal oxides from inert materials such as Zr, Al, Ca, B and Cd. Radioactive materials including the TRU elements and fission products (primarily Cs and Sr) comprise less than 1 wt% of the calcine. Aluminum and zirconium based calcines represent the greatest inventory of the calcine at approximately 20% and 80%, respectively. The average compositions of liquid tank waste and zirconium calcine are given in Table I. Radionuclides in the wastes that will require treatment and/or immobilization are U, Np, Pu, Am, Cs, Sr, and possibly Tc. Other hazardous constituents in the wastes that may require treatment are mercury, lead, chromium and cadmium.
Table I Average Compositions of Zr Calcine and Sodium Bearing Waste

In addition to the highly-radioactive wastes at the ICPP, several DOE sites have areas with groundwater that is contaminated with small amounts of radionuclides. One site at the INEL, the Test Area North (TAN), contains groundwater contaminated with volatile organic compounds, 90Sr, and 137Cs. Remediation efforts, mandated by CERCLA regulations, are underway at the TAN site. To support this activity, a number of sorbents have been evaluated to determine their efficiency at removing Sr and Cs from groundwater. Strontium-90 concentrations in the groundwater range from 200-500 pCi/L and cesium-137 concentrations range from 400-3000 pCi/L. The maximum acceptable contaminant level for the treated groundwater is 8 pCi/L for 90Sr and 119 pCi/L for 137Cs.
HIGH-ACTIVITY WASTE TREATMENT
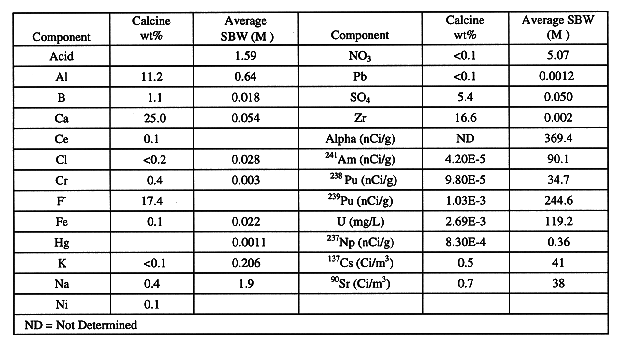
An aggressive development effort has been underway since 1992 to evaluate technologies for processing and immobilizing the liquid and calcine radioactive wastes. The recommended treatment method for the calcine is to dissolve it in nitric acid and then separate the radionuclides from the bulk waste components. The liquid tank waste will also be treated using the same processes. One flowsheet for separating the actinides, strontium and cesium from the aqueous acidic solutions (tank waste and dissolved calcine) is shown in Fig. 1. This flowsheet includes solvent extraction processes for the removal of actinides and strontium and an ion-exchange process for the removal of cesium. Alternative solvent extraction processes are under evaluation for actinide, strontium and cesium removal. The high-activity waste stream from the separation processes is concentrated and sent to the vitrification process. The decontaminated waste raffinates would meet NRC Class A LLW criteria and be immobilized as grout for near surface disposal.
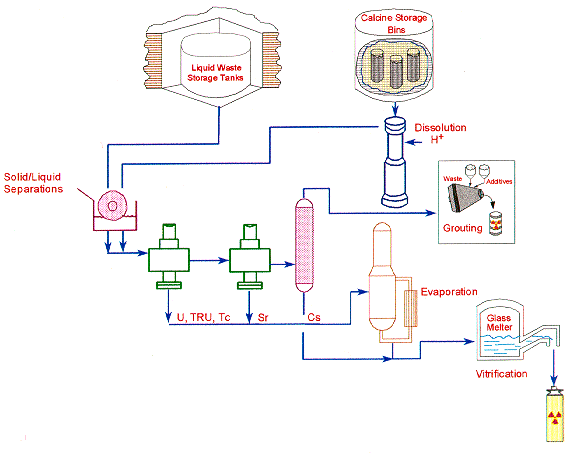
Fig. 1. Aqueous Separation Flowsheet
for ICPP Acidic Waste
The advantages offered by this flowsheet include: significant reduction of HLW volume, capability to process several waste streams in one facility, quick and efficient decontamination of aqueous waste streams, and production of a very consistent feed composition to the HLW vitrification process. The major disadvantage of this flowsheet is the additional capital cost required for the separations process, but analyses indicate this cost is more than offset by the reduction in the size and throughput of the HLW vitrification facility (1). Reduced HLW storage and disposal costs for the smaller HLW volume resulting from separations, would also result in substantial cost savings over directly immobilizing the INEL wastes.
Actinides (U, Np, Pu, Am) and Tc are removed from the aqueous waste streams by solvent extraction processes utilizing bifunctional neutral organophosphate compounds such as octyl (phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide (CMPO), dihexyl-N,N-diethylcarbamoylmethylphosphonate (DHDECMP) or phosphine oxide derivatives. The solvent extraction process based on CMPO as the extractant was originally developed by Horwitz and Schulz and called the TRUEX process (for TRansUranic EXtraction process). The TRUEX process, which uses a solvent comprised of 0.2 M CMPO and 1.4 M tributylphosphate (TBP) in a paraffinic hydrocarbon diluent, has been successfully demonstrated on both simulated and actual calcine and liquid tank waste (2,3,4). The TRUEX process has recently been demonstrated on actual waste in a 24-stage centrifugal contactor pilot plant which is installed in a remotely-operated hot cell facility at the INEL (5). Gross alpha activity of the waste was reduced from 457 nCi/g to 0.12 nCi/g. The NRC Class A LLW requirement is <10 nCi/g for the actinides. The TRUEX process has also been shown to effectively extract Hg which is easily removed from the solvent in the solvent wash process using sodium carbonate. The centrifugal contactor pilot plant located in the shielded hot cell facility is shown in Fig. 2.
Other potential actinide extractants, primarily DHDECMP and phosphine oxide derivatives, have been tested with simulated and actual tank waste. These solvents offer promising alternatives to the TRUEX process and will continue to be evaluated.
Fig. 2. Centrifugal Contactor Pilot
Plant in Shielded Hot Cell Facility
Strontium is selectively removed from the acidic raffinate from the TRUEX process by the SREX (StRontium EXtraction) process. The SREX process utilizes a crown ether extractant of 0.2M di-(tert-butylcyclohexo)-18-crown-6 (DtBuCH18C6) in 1-octanol (6,7,8). Recent test results indicate TBP in a hydrocarbon diluent is also an effective solvent for the crown ether (9). The SREX process has been demonstrated on simulated and actual tank waste and dissolved calcine. The tank waste presents the greatest challenge for this process as sodium and potassium, present in 4 to 5 orders of magnitude higher concentrations than strontium, will be partially extracted and reduce the amount of strontium extracted. Greater than 99.99% of the strontium was removed in six successive batch contacts with actual tank waste, using fresh solvent for each contact. A recent test performed in centrifugal contactors using simulated tank waste demonstrated effective removal of Sr and Pb from the waste and selective stripping of both the Sr and Pb into separate streams (10).
Three more important issues relative to the SREX process have been recently investigated. The first is the purity of the substituted crown ether extractant. Recent improvements in the isomeric purity of the crown ether by Eichrom Industries, Darien, IL, has increased the Sr distribution coefficient for 0.05 M crown ether in 1 M nitric acid from about 1.8 to 5 in the last few years. The second issue is the availability of the extractant in commercial-scale quantities. Eichrom Industries has developed the capability and maintains production in kilogram quantities. Third, the stability of the substituted crown ether in the SREX solvent has been demonstrated to 100 MRad levels of gamma radiation (11). These issues are significant for the future use of the SREX process on a production scale and indicate that the process has a high probability for successful implementation.
Cesium removal is accomplished in the flowsheet by ion exchange using inorganic sorbents. Numerous inorganic sorbents are available, but two have been successfully tested in columns with simulated and actual wastes. One sorbent is a copper-potassium hexacyanoferrate composite which is highly selective for Cs and can be regenerated for multiple cycles of cesium sorption. Testing of this particular sorbent, manufactured in Russia, was performed in a collaborative effort with scientists from the Institute of Physical Chemistry-Russian Academy of Sciences, Moscow, Russia (12). Another sorbent, IONSIV-911, which is manufactured by UOP, is also highly selective for Cs, but cannot be regenerated for multiple-cycle use. Both sorbents were tested with actual waste in a hot cell facility using a 1 cm3 column, shown in Fig. 3.
The method for disposition of the spent sorbent has not been determined. One option is to remove the spent sorbent, loaded with cesium, and send it to the vitrification process. It may be more economical to elute the cesium from the sorbent (if the cyanoferrate sorbent is used) and after 10-15 cycles of loading/elution remove the spent sorbent and treat it as a low-level waste. In this case, the cesium-rich eluent would be added to the actinide and strontium-rich streams prior to concentration/vitrification.
Fig. 3. 1 cm3 column in
shielded hot cell facility
A collaborative program between the Khlopin Radium Institute (KRI) of St. Petersburg, Russia and the INEL has resulted in testing of a chlorinated cobalt dicarbollide based solvent for the removal of strontium and cesium from ICPP wastes (13). Recent work at KRI has produced a non-aromatic solvent that would be acceptable for use in the United States. Testing of this process in centrifugal contactors was successfully performed with simulated waste in 1995 and with actual waste in 1996 (14,15).
A countercurrent flowsheet test was recently performed at Argonne National Laboratory using simulated INEL dissolved calcine with a solvent containing the primary crown ether extractant for the SREX process and a proprietary crown ether extractant which isselective for Cs. The test demonstrated excellent decontamination factors (>104) for Cs and Sr from INEL dissolved pilot plant calcines (16). The proprietary crown ether molecule, however, is not stable in nitric acid solutions for extended periods of time (several days).
The relative volumes of high-activity (HAW) and low-activity wastes (LAW) produced from the separations processes are illustrated in Fig. 4. The liquid waste shown (250 mL) represents current tank wastes. The volume of calcine (135 mL) is representative of calcine produced from the liquid wastes using recent process improvements and cold chemical addition to dilute the sodium in the waste to facilitate the fluidized bed calcination process. The volumes of the HAW glass (1.25 mL) and LAW grout (133 mL) are representative of the volumes that would be produced if the liquid were processed via separation processes rather than calcination. It is apparent that significant volume reductions in the high activity waste fraction can be realized by employing separation technologies. Relative glass volumes from the direct vitrification of calcines are typically 1.8-2.0 times the volume of the calcine.
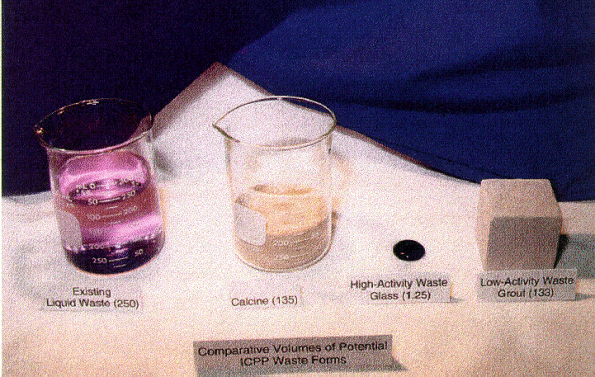
Fig. 4. Representative waste volumes
from processing of liquid tank wastes
GROUNDWATER DECONTAMINATION
The Groundwater Treatment Facility (GWTF) was constructed at the Test Area North (TAN) site on the INEL as an interim action for ground water remediation. The GWTF was started in 1994 as a pump and treat facility, primarily for the removal of volatile organics. The GWTF contained two ion-exchange columns for the removal of 90Sr. The columns were designed to be operated continuously at a rate of 50 gallons per minute and were originally loaded with Ionac C-250 resin. Decontamination of the water in the GWTF was inadequate due to limited capacity of the Ionac C-250 resin and the presence of other contaminants, namely 137Cs. Tests were performed with a number of sorbents to evaluate their effectiveness relative to the Ionac C-250.
Three ion-exchange column setups were installed at the GWTF and tested using a slip stream of water after organic removal and filtration. Two setups contained two columns, the first for cesium removal and the second for strontium removal. The first setup contained Pellx-137 in the first column, a zeolite which is selective for cesium, and Ionac C-250 in the second column for strontium removal. Ionac C-250 was tested as a control to validate the data with previous GWTF operating data. The second setup contained CsTreat in the first column, a commercial cobalt potassium ferrocyanide sorbent for cesium sorption, followed by sodium titanate, produced by Allied Signal, in the second column. The third setup was used with only one column containing IONSIV-911, a commercial product of UOP commonly referred to as crystalline silicotitanate. The IONSIV-911 sorbent is effective at removing both cesium and strontium at neutral pH. Testing was performed continuously whenever the GWTF was operating, which was typically about 2.5 days per week as it processed a single 38,000 L batch of water. All of the column setups were operated at 20 mLs/min (10 bed volumes/hr).
Another test setup was installed in the GWTF which contained the same type of cobalt potassium ferrocyanide sorbent used in the ion-exchange test, but engineered into a 3M web cartridge system. The 3M system is capable of processing high flowrates of water in a simple, compact system. The 3M system was operated on the same schedule as the ion-exchange column tests, but at a flowrate of 3.8 Lpm. The 3M system in this test only contained a web designed for cesium removal. Other materials which are selective for strontium have previously been tested in the 3M web system (17,18).
Approximately 175 liters (1456 bed volumes) of water were processed through the first ion-exchange system (Pellx-137/Ionac C-250). Cesium-137 activities were reduced to below 14 pCi/L over the duration of the test. Strontium-90 activities were reduced to about 6 pCi/L for most of the test, but strontium breakthrough began to occur at about 130 L (1100 bed volumes).
Approximately 228 liters (1900 bed volumes) of water were processed through the second ion-exchange system (CsTreat/Na Titanate). Cesium-137 activities were reduced to below 15 pCi/L over the duration of the test. Strontium-90 activities were reduced to levels between 13-56 pCi/L for the duration of the test. Breakthrough of cesium in the CsTreat sorbent was not observed. An increase in 90Sr activities in the column effluent was observed during the test, but it was not possible to determine definitively if breakthrough had occurred.
The IONSIV-911 column processed 171 liters (1430 bed volumes) of water. Cesium-137 activities were reduced to below 15 pCi/L over the duration of the test. Strontium-90 activities were reduced to less than 9 pCi/L for the duration of the test. Breakthrough of both cesium and strontium was not observed.
The 3M web system, containing a single 7.6 cm diameter by 25 cm tall ferrocyanide cartridge processed over 41,600 L of water. Cesium-137 activity was undetectable (<15 pCi/L) in the effluent from the 3M system for the duration of the test.
SUMMARY
Aqueous separation processes proposed for treatment of the two high-activity waste streams at the ICPP have been demonstrated to be feasible and cost effective. The waste streams can be decontaminated to levels well below the NRC Class A LLW criteria, allowing for near-surface disposal as grout. A strong emphasis is placed on performing laboratory and pilot-scale tests with actual wastes inside a shielded hot cell facility. Optimization of the separation processes to minimize high-level waste fractions and secondary wastes is in progress.
Several novel sorbents are now commercially available which offer improved selectivity and capacity for the removal of strontium and cesium from contaminated groundwater. Additional work is needed to obtain sufficient data to allow life-cycle cost analyses of using various sorbents in different engineered systems.
ACRONYMS
| CMPO | octyl (phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide |
| DHDECMP | dihexyl-N,N-diethylcarbamoylmethylphosphonate |
| DtBuCH18C6 | di-(tert-butylcyclohexo)-18-crown-6 |
| GWTF | Groundwater Treatment Facility |
| HAW | high-activity waste |
| HLW | high-level waste |
| ICPP | Idaho Chemical Processing Plant |
| INEL | Idaho National Engineering Laboratory |
| KRI | Khlopin Radium Institute |
| LAW | low-activity waste |
| LLW | low-level waste |
| MRad | Megarads |
| NRC | Nuclear Regulatory Commission |
| SREX | strontium extraction |
| TAN | Test Area North TBP tributylphosphate |
| TRUEX | transuranic extraction |
REFERENCES