Table I Tank C-106 Solids Waste Type, Volume, and Depth (Agnew et
al. 1996)
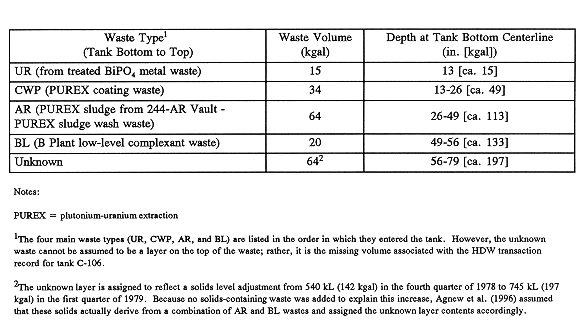
Fig. 1. Tank C-106
waste types.
R. D. Schreiber
Lockheed Martin Hanford Corporation
H. Babad
Lockheed Martin Hanford Corporation
R. J. Cash
Duke E&S Hanford Company
ABSTRACT
Single-shell tank C-106 is on an accelerated schedule for partial retrieval of its softer, high-heat sludge. The sludge is being transferred to a double-shell tank because they have the capacity to handle more heat-bearing materials than do single-shell tanks. Also, unlike single-shell tanks, they have not shown any tendency to leak. This transfer will eliminate the need to add water to tank C-106, thus lowering the risk of waste leaking to the environment. The transfer also will allow obligations to the Washington State Department of Ecology regarding removal of drainable liquid from all single-shell tanks to be met. Current schedules show the soft-sludge retrieval starting in September 1997. To prepare for retrieval, issues related to the risk from potential propagating reactions caused by the organic chemistry of tank C-106 were evaluated.
INTRODUCTION
Tank C-106 is the only single-shell tank on the Hanford Site where the high heat generated by the stored waste is a concern. Based on its capacity to passively store waste, this tank received an excess of sludge containing high levels of strontium-90. To prevent structural damage to the tank, evaporative cooling is used to maintain its temperature within safe operating limits. This is done by periodically adding water to the tank and allowing the water to evaporate. However, because 67 of the 149 single-shell tanks on the Hanford Site are assumed leakers, this method of maintaining temperature control is an unsatisfactory compromise between waste management and environmental protection. Without repeated water additions, which would need to be discontinued if the tank began to leak, the tank could exceed structural temperature limits, potentially causing a dome collapse. Because present retrieval methods will not recover all of the tank's waste, the safety of the residues remaining after partial retrieval and dryout needed to be defined.
THE CHEMISTRY AND STRATIGRAPHY OF TANK C-106
Tank C-106 has the capacity to store approximately 2,000-kL (530-kgal) of waste. The tank currently stores 867kL (229kgal) of waste. This waste is separated into four major identifiable layers. The bottom layer consists of 186 kL (49 kgal) of uranium recovery (UR) and Plutonium-Uranium Extraction (PUREX) coating (CWP) wastes, called the "hardpan." The remaining 560kL (148kgal) of solid waste is high-heat, higher plutonium- and strontium-containing, soft sludge from B Plant and AR Vault. Finally, a 121kL (32kgal) layer of supernate is located on top of the soft sludge.
Based on alternative interpretations of historical data, different waste layer volumes may be found in some source documents. However, this difference has no effect on the outcome of the studies reported here.
The waste contents of tank C-106 are described in Table I and are illustrated in Fig. 1.
Table I Tank C-106 Solids Waste Type, Volume, and Depth (Agnew et
al. 1996)


Fig. 1. Tank C-106
waste types.
The hardpan layer in tank C-106 consists of UR (formally called tributyl phosphate [TBP] waste) and CWP waste, as shown in TableI. The CWP layer consists of cladding waste additions from PUREX operations accumulated through the second quarter of 1960.
The AR layer of waste consists of solids that were transferred from AR Vault to tank C-106 from 1967 to 1971. During this operation, PUREX sludge solids that were sluiced from A and AX farms were fed to the AR Vault and allowed to settle. The supernatant was transferred to tank C-106, allowed to clarify, and then transferred to tank C-105 as feed for the cesium recovery process. Low-cesium supernatants from tank C-105 were then cycled back to the AR Vault for caustic sludge washing to leach as much cesium out as possible. These washings were then cycled back to tank C-105 through tank C-106.
The AR solids that settled out were then acid digested and the supernatant from that process was sent to B Plant where the strontium was removed. Any remnant solids were reneutralized and recycled through the strontium recovery process. The AR solids that accumulated in tank C-106 and other tanks were derived from peptized (non-sedimented) solids from these processing activities.
In 1974, as a result of an attempt to move some of the AR solids to other C Farm tanks by pumping, some AR solids from tank C-106 were moved to tank C-103. At that point, tank C-106 began receiving B Plant low-level complexant (BL) waste; the upper layers of the tank consist of these additions. The tank was declared inactive in early 1979.
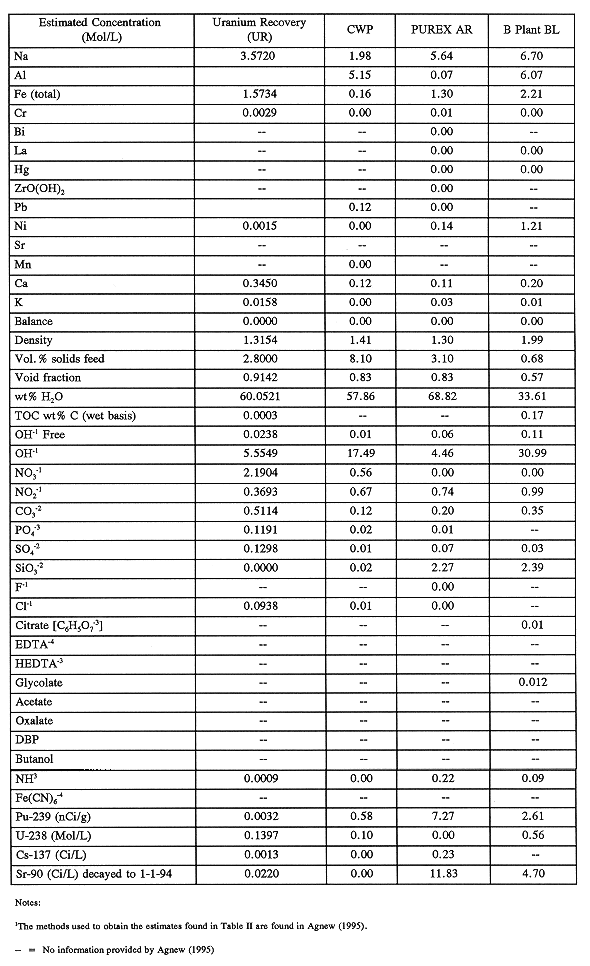
Once again, the unknown 240-kL (64-kgal) layer is most likely AR and BL solids that were not accounted for. Agnew (1995) has treated them as such in his inventory prediction. His Hanford Defined Waste layer compositions for these waste types are listed in Table II.
Table II Predicted C-106 Waste Chemistry Composition from HDW
Estimate 91)
A combination of sample analysis and computation has demonstrated that the plutonium concentration in tank C-106 is still well below concentrations of criticality concern (Waltaretal. 1996; Whyatt et al. 1996).
ORGANIC CHEMICAL CONCENTRATION CONCERNS
A key safety issue is whether tank C-106 contains sufficient organics to pose a risk for propagation after the soft sludge is removed, if the tank contents are allowed to dry out. The main reason for retrieving part of the soft sludge from tank C-106 is to reduce the heat load so that cooling water additions are no longer needed. If sufficient waste is removed the need for active ventilation of the tank will also be eliminated. The addition of water does not, in itself, pose a potential for a loss of tank integrity. However, in single-shell tanks, which already have a potential for leaking, adding water creates the potential for a larger discharge to the soil if the tank begins to leak. Such a leak is not unlikely; 67 single-shell tanks have been classified as assumed leakers and have required saltwell pumping to reduce the drainable liquid inventory that could be leaked to the soil.
The organic safety program considers a tank at risk from a propagating reaction if that tank contains 3wt% or more total organic carbon (TOC) with an energy value of at least 480 J/g [dry weight basis] (Turneretal. 1995). The presence of water mitigates these conditions somewhat, but cannot be relied on exclusively in high-heat tank C-106. Also, tanks containing significantly less organic (0.8 to 3wt% TOC), if the organic is associated with species that contain carbon-hydrogen and nitrogen-hydrogen bonds, generate hydrogen gas at higher rates than the rate for radiolysis of water. The presence of hydrogen causes a potential flammable gas safety issue.
The Safety Screening data quality objective (DQO) (Dukelow et al. 1995) requires testing of tank samples for energetics, as well as for moisture content. If energetics of 480J/g (dry weight basis) are found, a secondary analysis for TOC is required. Because of the interest in the risk from organics, TOC analysis was made a primary analysis in the sampling and analysis plan (Schreiber 1996a). Standard characterization practices were further modified to add a dewatering step. The dewatering step helps to minimize analytical ambiguities and identifies species-specific effects between the tank solids and the aqueous solutions in the waste. The step would allow one to determine whether energetics in a waste sample were caused by fuel-rich soluble organic complexants that could be easily removed by sluicing or whether they were associated with the solids. This question focuses on the broader issue of how completely the soft materials in tank C-106 could be sluiced, an issue evaluated in the Project W-320 Safety Assessment (WHC 1996).
The analytical laboratory was asked to dewater the sludge by centrifugation using a filter cone to maximize the separation of water-soluble and -insoluble waste components. During centrifugation, a previously unencountered sludge-associated organic oil also separated out. The organic oil or "organic layer" floated on the aqueous layer. When the organic oil was observed, the process for dewatering the bulk of the sludge was altered. A centrifugation step was added to remove the organic oil and most of the liquid before the sludge was centrifuged through the filter cones. This "pre-centrifugation" step produced 0.2 to 0.5 mL of organic layer for each 50-mL sample of raw wet sludge.
Table III contains the results of differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and TOC analysis of the sludges obtained from tank C-106. The data are harder to interpret than usual because the dewatering step required in the sampling and analysis plan added an unexpected complication to the analysis and interpretation process.
Table III Organic Related Analysis: Average DSC, TGA, and TOC
Sludge Sample Results by Waste Depth (1)
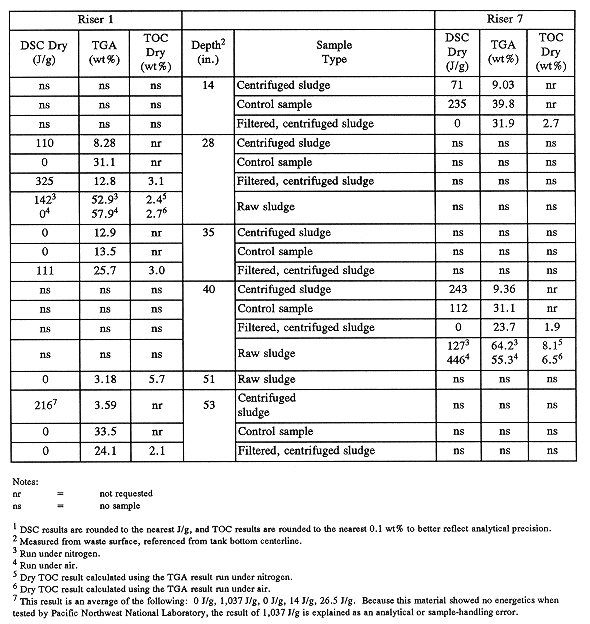
As can be seen from Table III, the dewatered sludge samples obtained from tank C-106 were moderately high in organics (some samples >3wt% TOC), but relatively low in energy. This is in keeping with the aging of B Plant organics to sodium oxalate, which is insoluble in the tank waste. Speciation of sludge samples confirmed the presence of significant concentrations of sodium oxalate (see Table IV).
Table IV Average C-106 Sludge TOC and Oxalate Results by Waste Depth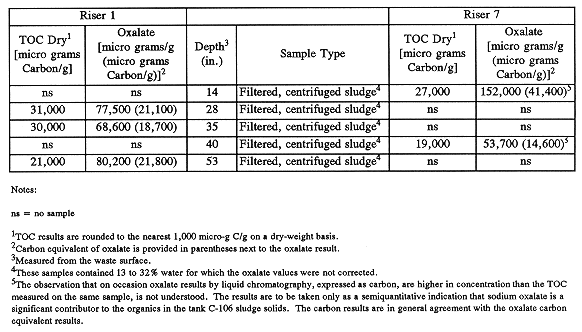
One anomaly observed in comparing TOC and oxalate analysis results was that the oxalate analysis procedure sometimes resulted in a higher apparent concentration of sodium oxalate than is bounded by TOC analysis. Because sodium oxalate is quantitatively degraded to carbonate by persulfate oxidation, this anomalous result suggests a matrix interference in the oxalate assay, perhaps another analyte eluting under the oxalate peak.
Speciation of the aqueous fractions produced during the solid-liquid separation (dewatering) steps showed that these fractions contain only small amounts of soluble complexant compared to their carbon content.
POTENTIAL FOR A PROPAGATING REACTION OF THE RESIDUAL ORGANICS IN TANK C-106 AFTER DRYOUT
The waste in tank C-106 consists of the following types from top to bottom of the tank:
Only BL waste should contain organic complexants. Agnew (1995) estimated in his model that much of the organic added to tank C-106 was citrate. However, the B Plant flowsheet indicated that most of the citrate in the waste stream was destroyed by the B Plant evaporator, so the actual carbon-containing species would be citrate degradation products (e.g., oxalate and carbonate).
This historical information, coupled with the data reported earlier, suggests that the organics in tank C-106 are both well-aged (with large oxalate concentrations) and energetically benign. Therefore, leaving some or all of the organics in tank C-106 in the absence of evaporative cooling will not pose a risk of a propagating organic reaction.
THE COMPOSITION OF THE OIL RELEASED BY SLUDGE CENTRIFUGATION
To maximize the information obtained from the tank C-106 samples, an extensive dewatering step was built into the laboratory test plans (Schreiber 1996a). The laboratory staff were asked to dewater the sludge in a centrifuge using a fritted disk or filter cone to maximize separation of water-soluble and -insoluble components. This step was designed to avoid anomalies sometimes observed when samples containing more than 40 percent water are analyzed. Errors in analysis results are of particular concern when waste samples contain species (analytes) that partition in both the aqueous and solid phases. Standard centrifugation in a tapered cone was also performed on the sludge samples. These centrifugation steps resulted in the separation of a previously unencountered, sludge-associated organic oil that floated on the aqueous waste layer. The results of speciation and other tests with the sludge oil from tank C-106 are described in the following paragraphs.
Summary of Findings
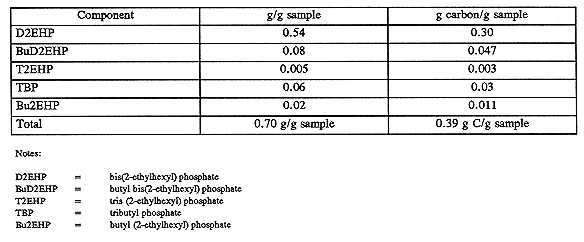
Two of the oil samples centrifuged from tank C-106 sludge, identified as 7-SA and 13-3, were submitted to Pacific Northwest National Laboratory (PNNL) for organic speciation. Using a combination of infrared (IR) spectroscopy, gas chromatography/mass spectrometry (GC/MS), and liquid chromatography, PNNL scientists identified the various constituents of the oil, achieving a carbon accountability of nearly 80% for the process. The principal constituent of the oil was the compound bis (2-ethylhexyl) phosphoric acid, existing as sodium bis (2-ethylhexyl) phosphate in the waste. Minor amounts of TBP, normal paraffin hydrocarbon, and the transesterification products of TBP and 2-ethylhexyl alcohol, or di(2-ethylhexyl) phosphate and butyl alcohol also were present.
Sodium bis (2-ethylhexyl) phosphate, the salt of bis (2-ethylhexyl) phosphoric acid, was used as a complexing agent in B Plant during the strontium recovery campaigns. This salt likely coprecipitated with the sludge when waste from B Plant was made alkaline before being transferred to the tanks. The absence of a strongly alkaline environment in tank C-106 likely protected the salt from hydrolysis or, like sodium bis-dibutyl phosphate, it may resist alkaline hydrolysis. However, Camaioni et al. (1996) demonstrated that sodium bis-dibutyl phosphate is readily destroyed under radiolytic conditions; therefore, the survival of sodium bis (2-ethylhexyl) phosphate in tank C-106 remains unexplained.
DSC and TGA analyses of the isolated oil and propagation tests with authentic sodium bis (2-ethylhexyl) phosphate showed that the oil would not result in nitrate-nitrite-induced propagation reactions when heated to 300°C with a stoichiometric amount of oxidizer (Fauske 1996a and 1996b). Similar evidence of chemical inertness has been found for other phosphate esters and salts in the nitrate-nitrate oxidizer system typical of Hanford Site tanks. The analysis results for sample 7-SA and 13-3 are presented in Tables V and VI, respectively.
Table V Analysis of Sample 7-SA, Riser 1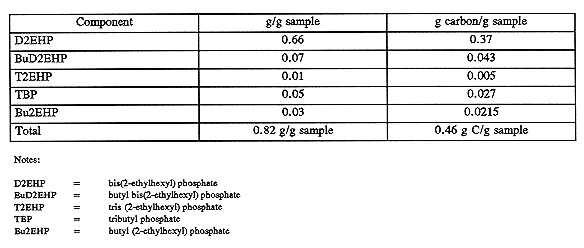
Table VI Analysis of Sample 13-3, Riser 7
CONCLUSIONS
Although tank C-106 contains precipitated carbon compounds, these species are not energetic in nature. The carbon in the solid fraction of the waste was found to be predominantly sodium oxalate with a small amount of sodium bis (2-ethylhexyl) phosphate. These materials pose no threat if, after retrieval of the high heat fractions of the waste, the tank is allowed to dry out.
Additional information can be found in the detailed tank characterization report prepared for tank C-106 (Schreiber 1996b).
REFERENCES