Fig. 1. Excess Neutron Absorber
Content in Waste Streams Basedon Flowsheet Composition.
D. R. Bratzel
Duke Engineering and Services Hanford,
Inc.
P.O. Box 350
Richland, Washington 99352
(509) 373-3579
C. L. Sohn
U.S. Department of Energy
P.O. Box 550
Richland, Washington 99352
(509) 376-8523
R. J. Serne
Pacific Northwest National Laboratory
P.O. Box 999
Richland, Washington 99352
(509) 376-8429
W. W. Schulz
W2S Company
5314 Arustos Court, NE
Albuquerque, New Mexico 87111
(505) 299-4854
R. Vornehm
7008 Regency Road
Knoxville, Tennessee
37931
(423) 938-1883
H. Babad
Babad Technical Services
2540 Cordoba
Court
Richland, Washington 99352
(509) 375-0328
ABSTRACT
This paper describes the approach used to resolve the Nuclear Criticality Safety Issue for the Hanford Site high-level waste tanks. Although operational controls have been in place at the Hanford Site throughout its operating life to minimize the amount of fissile material discarded as waste, estimates of the total amount of plutonium that entered the waste tanks range from 500 to 1,000kg. Nuclear criticality safety concerns were heightened in 1991 based on a review of waste analysis results and a subsequent U.S. Department of Energy (DOE) review of the nuclear criticality program. Although the DOE review team concluded that there was no imminent risk of a criticality at the Hanford Site tank farms, the team also stated its concern regarding the lack of definitive knowledge of the fissile material inventory and distribution within the waste tanks and the lack of sufficient management support for the overall criticality safety program.
An in-depth technical review of the nuclear criticality safety of the waste tanks was conducted to develop a defensible technical basis to ensure that waste tanks are subcritical. The review covered all relevant aspects of nuclear criticality safety including neutronics and chemical and physical phenomena of the waste form under aging waste conditions as well as during routine waste management operations. This paper provides a review of the technical basis to support the conclusion that given current plutonium inventories and operating conditions, a nuclear criticality is incredible. The DOE has been requested to close the Nuclear Criticality Safety Issue. The Defense Nuclear Facilities Safety Board is currently reviewing the technical basis.
Since the early 1940's, a significant quantity of high-level radioactive waste, including the fissile isotopes of plutonium and uranium, has been generated from defense operations and stored in the Hanford Site underground waste storage tanks. Because the mission of the Hanford Site was to maximize plutonium output for defense purposes, there was a strong incentive to minimize the amount of this material discarded as waste. The best estimate for the total amount of plutonium that entered the Hanford Site waste tanks is about 500kg although some estimates are as high as 1,000kg because of uncertainties in some measurements (Bratzel etal.1996).
Operational controls have been in place throughout the history of the Hanford Site production plants to ensure fissile material concentrations in the waste streams are subcritical before transfer to the underground storage tanks. Historically, the fissile material concentration in the wastes has been controlled at a level that would make the initiation of a self-sustaining nuclear chain reaction (a criticality accident) incredible. This belief has been based on the assumption that the fissile components of the waste remain at a relatively low concentration and are relatively well mixed with the bulk of the oxyhydroxide neutron absorbers.
In June 1991, a waste sample indicated that the sampled tank might have a total fissile material content greater than the criticality prevention specification limit (the value was subsequently determined to be erroneous due to a calculational error). A U.S. Department of Energy (DOE) review team concluded that although there was no imminent risk of a criticality at the Hanford Site tank farms, there was a lack of definitive knowledge of the fissile material inventory and distribution within the tanks and a lack of management support for the overall criticality safety program.
In April 1992, a review of the existing nuclear criticality safetyreports concluded that because the safety envelope was inadequately defined by existing analyses, an inadequacy existed in the authorization basis and an unreviewed safety question (USQ) was declared. The nuclear criticality USQ was closed in 1994 after an evaluation of the available waste tank and process data determined that the plutonium concentration in the waste was subcritical (Braunetal.1994). However, the safety issue remained open because of additional technical concerns of whether the fissile material remained associated with the neutron absorbers during waste aging over time as well as under ongoing waste management operations. The residing safety concern has been whether chemically or physically induced gradients may result in the separation and concentration of plutonium from the neutron-absorbing oxyhydroxide solids, resulting in an accidental nuclear criticality.
An in-depth technical review of the nuclear criticality safety of the waste tanks has been conducted. The review covered all relevant aspects of nuclear criticality safety including waste tank sample analysis results; the neutronics of the waste tank system; and chemical, physical, and hydraulic factors. These factors were evaluated relative to initial deposition of the wastes in the tanks, during aging of the wastes, and during established operating conditions. The results of the technical review confirm previous conclusions, which state that a nuclear criticality is incredible under current plutonium inventories and operating conditions (Bratzel et al. 1996). This technical review has been incorporated into the authorization basis for the continued safe operation of the tank farm facility. The foundation of the tank farm facility criticality safety program is the double-contingency principle. Continued criticality safety in the tank farms is dependent upon the waste generators verifying compliance with the tank farm criticality safety limits and controls. These limits and controls ensure transfers of waste are limited to subcritical plutonium concentrations and contain an excess of neutron absorbers.
WASTE TANK CRITICALITY CONDITIONS
Waste tank nuclear criticality is a function of four important factors:
The first two parameters are particularly important with respect to prevention of an accidental criticality of the tank waste contents. The primary defense to ensure subcriticality in the waste tanks is based on insufficient concentration of the fissile material in the presence of the neutron-absorbing solids. Operational limits and controls to ensure subcritical plutonium concentrations in the waste streams have been the only defendable limit and control applied during the operational history of the Hanford Site production and waste storage tank facilities. However, many other factors provide an additional margin of subcriticality. The variability in tank-to-tank waste composition, the difficulty in obtaining representative tank waste samples, and the lack of historical operational control make it impractical to take quantitative credit for these factors. They do, however, contribute qualitatively to the overall safety basis.
Fissile Material Quantity and Concentration
Fissile material consists of the odd-numbered isotopes of uranium and plutonium. The fissile material content of the waste is expressed in terms of its 239Pu equivalence. For the Hanford Site tank waste, the plutonium concentration must be at least 2.6g Pu/L before criticality becomes possible, even in an unlimited volume of waste (Rogers1996). This value is based on a uniform mixture of plutonium, waste solids, and water as described in the Hanford Site Conservative Waste Model (CWM) (Rogers1993). The CWM is based on the neutronic characteristics as well as the chemical constituents of the waste which act as effective neutron absorbers. The CWM defines the waste composition by using the maximum reported value for solids materials that are good neutron reflectors and the minimum reported values for solids materials that are good neutron absorbers. The CWM also assumes the water content is optimized. The use of this CWM results in a minimum subcritical plutonium concentration value that is well below the value of 7.2g/L, which is generally reported as the minimum critical concentration of plutonium in pure water.
Plutonium concentration has historically been used as the primary control on waste streams discharged to the storage tanks (Serneetal.1996). Documents and procedures from the mid-1960's indicate control of discharge levels at a maximum of 0.013g Pu/L in a waste mixture being routed to a waste tank and 1g Pu/L in waste tank solids (Braunetal.1994). Reviews of flowsheets and waste transfer documents produced before 1960 indicate that this level of control has always existed. A statistical analysis of the extensive number of waste tank characterization samples to date (more than 500) confirms the effectiveness of the concentration controls (Serne et al.1996).
Collectively, the single-shell tanks (SST) and double-shell tanks (DST) contain an estimated 500 to 1,000kg of plutonium. Analyses of tank waste samples clearly establish that the plutonium content of the waste in any tank is associated almost exclusively with the sludge phase. The maximum measured plutonium concentration in a sludge phase is about 0.35 to 0.7 g Pu/L. This maximum measured concentration is a factor of 4 to 7 below the minimum subcritical concentration of 2.6gPu/L conservatively derived for tank waste conditions. In most of the SSTs and DSTs, the plutonium concentration in the sludge phase is at least 100 times less than 2.6gPu/L. These conditions reflect the deliberate controls exercised throughout operation of fuel reprocessing and purification facilities to maintain the plutonium concentration in waste streams to very low levels.
If the plutonium areal density does not exceed 2,582g/m2, subcriticality is ensured in the Hanford Site waste tanks (Rogers 1996). This value is independent of the plutonium concentration and will increase if waste solids (i.e., neutron absorbers) are taken into account. Taken into perspective, the entire plutonium inventory from all 177 Hanford Site storage tanks could be stored in a single tank without exceeding subcritical conditions assuming that the plutonium is distributed with relatively lateral homogeneity.
Other Nuclear Criticality Factors
Although operational controls to minimize the plutonium concentration are the primary defense to ensure subcriticality in the waste tanks, several other factors provide an additional margin of subcriticality.
Neutron Absorbers: Abundant analytical data show that plutonium in SST and DST sludges is intimately associated with large amounts of iron, manganese, chromium, and other metals that are good neutron absorbers. These metals precipitated along with plutonium when initially acidic wastes were made alkaline before being introduced into the waste tanks. The neutron absorber(s)-to-plutonium mass ratios are typically well above those needed to ensure subcritical conditions.
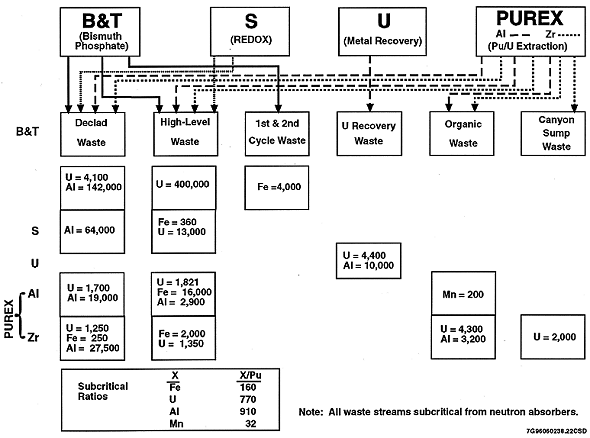
Figure 1 is a summary graphic of the neutron absorber-to-plutonium ratio for each Hanford Site process waste stream entering the waste tanks based on flowsheet composition. Shown below each of the waste streams are the absorber-to-plutonium ratios (X:Pu) based on the flowsheet composition for each waste stream. The minimum ratios necessary to ensure subcriticality for each absorber type are summarized at the bottom of the graphic. This figure illustrates that each waste stream contains sufficient neutron absorbers of at least one type (if not more) to maintain a subcritical composition, irrespective of any other neutronic condition.

Fig. 1. Excess Neutron Absorber
Content in Waste Streams Basedon Flowsheet Composition.
Neutron Moderation: Water (i.e., hydrogen atoms) moderates neutrons, reducing their energy to thermal levels. Water also serves as a neutron absorber when in excess of some amount (optimum moderation). The stored tank wastes are, in general, over-moderated, which adds to the conservatism of the 2.6gPu/L minimum subcritical concentration criterion specifically derived for the Hanford Site waste tanks (Rogers 1996).
Geometry: Although the most likely configuration of the sludge (which contains the majority of the fissile material) in the Hanford Site storage tanks is a slab-like layer, the waste form can also exist as other shapes (e.g., conical) from the waste addition process. At a concentration greater than 2.6gPu/L, well above the maximum observed waste concentration, a slab can be made critical. However, large amounts and higher concentrations of plutonium (e.g., 5,000kg at 3gPu/L, 1,500kg at 6gPu/L) would be required to attain criticality, and these are unachievable under current waste storage conditions. Regardless of the shape of the waste form, safety analysis calculations typically assume a sphere geometry (the most conservative shape from a neutronics perspective) to ensure they sufficiently bound actual waste tank conditions.
CHEMICAL AND PHYSICAL PROCESSES AND FLUID DYNAMICS
The neutronics of the waste system show that the waste contents are subcritical based on a subcritical plutonium concentration and presence of a sufficient concentration of neutron absorbers in the waste streams upon addition to the tanks. To address safety concerns of whether the fissile material could be separated and concentrated from the neutron absorbers as the waste ages and during normal waste tank operations, a technical basis has been established to evaluate the chemical and physical processes and fluid dynamics of the respective processes.
Chemistry of the Waste Form
Waste management practices always required that liquid waste streams generated in the production plants be neutralized with sodium hydroxide before transfer to the underground tanks. Addition of sodium hydroxide not only precipitated fissile materials in the waste but also co-precipitated various nonradioactive metal cations such as iron and other effective neutron absorbers as oxyhydroxides. The fine particulate solids that precipitated from the alkaline solution settled slowly in the waste tanks. These waste management practices have resulted in the vast majority of the plutonium and other fissile materials being associated with the neutralized sludge fraction of the waste because plutonium, iron, and other metals exhibit low solubility in the alkaline tank supernatant liquid. Thus, the focus of the technical basis analysis is on the in-tank physical and chemical behavior of the sludge fraction.
Three potential chemical mechanisms whereby plutonium entered the sludge phase have been identified:
If sorption were the dominant chemical mechanism, bonding of plutonium to strong neutron-absorbing metals such as iron and manganese ensures subcritical conditions even during sludge resuspension and settling. If pure hydrous plutonium oxide formed as a crystalline precipitate or if select solid-solid solution phases formed when acidic wastes were made alkaline, the plutonium-bearing particles would be expected to agglomerate or mix with neutron absorbers. Because ionic radii of Pu(IV), Zr(IV), La(III), and Bi(III) are quite similar, it is possible that some solid solution formation occurred in wastes containing these ions (e.g., zircaloy-cladding waste and bismuth process waste). However, the bismuth process waste solutions also contained large quantities of iron (a neutron absorber) so some of the plutonium also could have co-precipitated with hydrated iron oxide. Also, in the zircaloy-cladding wastes, the zirconium-to-plutonium mass ratios exceeded the minimum ratio needed to ensure subcriticality. Therefore, the plutonium in all waste streams (regardless of the precipitated chemical form) was associated with strong neutron-absorbing metals to ensure subcriticality.
For nuclear criticality to occur during mechanical mixing for either the pure plutonium oxide or plutonium solid-solid solution forms, large (tens of micrometers in size) plutonium particles free of neutron absorbers must be created and then concentrated by some mechanism. All literature data, as well as calculations performed for modeling operational mechanisms such as salt well pumping, air lift circulators, and transfer pumps, indicate that separation of such plutonium-bearing particles is not plausible.
Any chemical mechanism for concentrating the plutonium in a waste tank would require transport of plutonium from the solid phase to the aqueous liquid phase and then to a concentrated small volume solid phase. The supernate solutions have high concentrations of dissolved nitrite that act as a mild reducing agent, keeping the dissolved plutonium primarily as Pu(IV) which is more insoluble than Pu(V) or Pu(VI). Plutonium sorption on oxyhydroxides is considered to be mostly irreversible under the highly alkaline tank conditions (Serne1996). To drive the plutonium into the soluble phase, the addition of extremely large quantities of complexants, change in pH, or a change in the reduction-oxidation potential would be required. Because all additions to the tanks are controlled, this is not a reasonable possibility. Aging processes, including radiolysis, were found incapable of creating significant changes in these chemical variables.
Fluid Dynamic Processes
Calculations and modeling have been performed to address the following effects:
All literature data, as well as calculations for modeling hydraulic operations, indicate that separation and concentration of plutonium-bearing particles are insignificant (maximum enrichment factor <3) in comparison to the separation factors needed (>20). Thus an accidental criticality cannot occur as a result of waste aging or operational activities. As a result, if no waste were ever added to that which presently exists, a criticality accident is incredible.
SUMMARY AND CONCLUSIONS
Figure 2 is a summary graphic representation of the significant margin of subcriticality of the waste. The graphic compares actual waste parameters and operating limits (lower left corner) to calculated subcritical concentration limits as identified by the two curves. The two subcritical concentration curves show the degree of conservatism that has been included in the criticality safety analysis of the Hanford Site tank wastes. The "optimized conservative waste model (CWM)" subcritical concentration represents conservatively modeled waste conditions. The "average waste composition" represents subcritical concentrations for a waste composition representing an average composition of 19 elements from 28 tank samples. All cases are calculated using additional conservatism (e.g., assumes a sphere geometry). Figure 2 shows that even with a high degree of conservatism, the waste tank contents are highly subcritical.
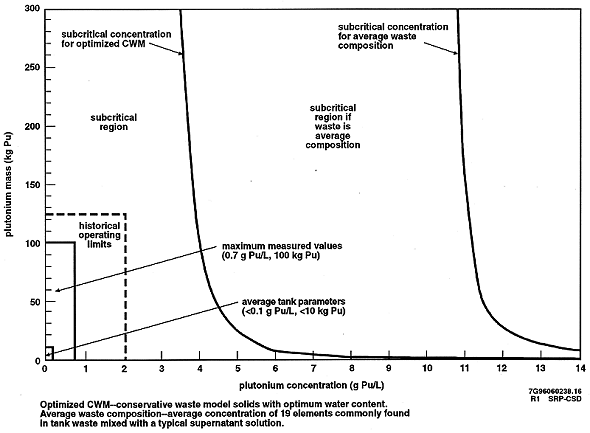
Fig. 2. Plutonium Mass versus Concentration--Perspective of Hanford Site Waste Tank Subcritical Concentrations (Spherical Geometry)
In conclusion:
BIBLIOGRAPHY
BRATZEL, D. R., W. W. SCHULZ, R. VORNEHM, 1996, Tank Farm Nuclear Criticality Review, WHC-SD-WM-TI-725, Rev. 0, Westinghouse Hanford Company, Richland, Washington.
BRAUN, D. J., L. D. MUHLESTEIN, T. B. POWERS, and M. D. ZENTNER, 1994, High-Level Waste Tank Subcriticality Safety Assessment, WHC-SD-WM-SARR-003, Rev.0, Westinghouse Hanford Company, Richland, Washington, pp. B-9 to B-20.
Rogers, C. A., 1996, CSER 96-010: Criticality Parameters for Tank Waste Evaluation, WHC-SD-SQA-CSA-507, Rev. 0, Westinghouse Hanford Company, Richland, Washington.
ROGERS, C. A., 1993, CSER 92-009: An Analytical Model for Evaluating Subcritical Limits for Waste in Hanford Site Storage Tanks, WHC-SD-SQA-SA-20356, Rev. 0, Westinghouse Hanford Company, Richland, Washington.
SERNE, R. J., G. A. WHYATT, S. V. MATTIGOD, Y. ONISHI, P. G. DOCTOR, B.N. BJORNSTAD, M. R. POWELL, L. M. LILJEGREN, J. H. WESTSIK, N.J. AIMO, K. P. RECKNAGLE, G. R. GOLCAR, T. B. MILEY, G. R. HOLDREN, D.W. JEPPSON, R. K. BIYANI, and G. S. BARNEY, 1996, Fluid Dynamic Particulate Segregation, Chemical Processes, and Natural Ore Analog Discussions that Relate to the Potential for Criticality in Hanford Tanks, WHC-SD-WM-TI-757, Rev. 0, Westinghouse Hanford Company, Richland, Washington.