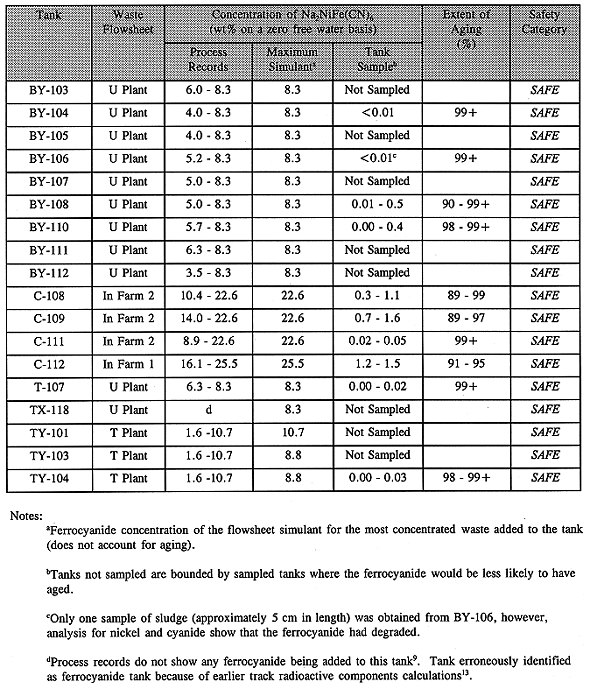
Table I Comparison of Tank Contents with Tank Safety Criteria
R. J. Cash , J. E. Meacham
DE&S Hanford, Inc.
P.O. Box 350
Richland, Washington 99352-0350
M. A. Lilga
Pacific Northwest National Laboratory
P.O. Box 999
Richland, Washington 99352-0999
H. Babad
Babad Technical Services
2540 Cordoba
Court
Richland, Washington 99352
ABSTRACT
The Ferrocyanide Safety Issue at the Hanford Site was officially resolved in December 1996. This paper summarizes the key activities that led to final resolution of this safety hazard, a process that began in 1990 after it and other safety concerns were identified for the underground high-level waste storage tanks at the Hanford Site. At the time little was known about ferrocyanide-nitrate/nitrite reactions and their potential to cause offsite releases of radioactivity.
The ferrocyanide hazard was a perceived problem, but it took six years of intense studies and analyses of tank samples to prove that the problem no longer exists. The issue revolved around the fact that ferrocyanide and nitrate mixtures can be made to explode violently if concentrated, dry, and heated to temperatures of at least 250°C. The studies conducted over the last six years have shown that the combined effects of temperature, radiation, and pH during 40 or more years of storage have destroyed almost all of the ferrocyanide originally added to tanks. This was shown in laboratory experiments using simulant wastes and confirmed by actual samples obtained from the ferrocyanide tanks. The tank waste sludges are now too dilute to support a sustained exothermic reaction, even if dried out and heated to high temperatures.
INTRODUCTION
During the 1950s, production of plutonium for defense created a need for additional waste storage space. To obtain this space quickly, while minimizing construction of new tanks, Hanford Site scientists developed a process to remove (scavenge) radiocesium (137Cs) and other soluble radionuclides from tank waste liquids through precipitation processes. Sodium ferrocyanide, followed by nickel sulfate, was added to the waste to create a nickel ferrocyanide precipitate that carried down the soluble 137Cs. The slurry containing the solids was transferred to single-shell, high-level radioactive waste tanks for settling. After seven to 10 days, the supernatant was decanted to the ground via cribs and trenches.
This scavenging process lasted from 1953 until early 1958, and used approximately 140 metric tons (154 tons) of ferrocyanide that ended up in 18 tanks. Ferrocyanide was added in excess; hence, the major precipitate was sodium nickel ferrocyanide [Na2NiFe(CN)6]. Ferrocyanide, at sufficiently high concentrations and mixed with sodium nitrate/nitrate oxidizers also present in the tanks, can be made to react exothermically by heating it to high temperatures. The release of energy comes from oxidation of the carbon-nitrogen triple bond; thus the form of the ferrocyanide (or cyanide) species present makes little difference in the energy released. A unique feature of the scavenging process was the addition of nickel, a distinctive cation that identifies whether the correct locations in the tank were sampled. No other significant source of nickel was added to the waste tanks at the Hanford Site.
In 1990, little was known about the conditions required to start a ferrocyanide-nitrate/nitrite reaction in Hanford Site tanks and the consequences that might result. Because of the heightened concern about ferrocyanide and another Hanford problem--episodic releases of flammable gas from several tanks, a Waste Tank Safety program was started in September1990 to address these important issues. Both problems were identified as Priority one safety issues by the U.S. Department of Energy (DOE). Because the existing safety documentation did not adequately address a ferrocyanide accident, an unreviewed safety question (USQ) was declared in October. In the same month the Defense Nuclear Facilities Safety Board (DNFSB) issued Recommendation 90-7, which asked DOE to provide enhanced monitoring and surveillance of the ferrocyanide tanks, sampling of the tank waste to determine if it could react, and chemical reactivity studies to evaluate ferrocyanide reactions. A General Accounting Office study (1) was also released in October 1990 that postulated a "worst-case" accident with offsite doses one to two orders of magnitude greater than stated in the 1987 environmental impact statement(2).
Public Law 101-510, Section 3137, "Safety Measures for Waste Tanks at Hanford Nuclear Reservation," was enacted in November 1990. This law required the identification of Hanford Site "Watch List" tanks that may have a serious potential for release of high-level waste. The ferrocyanide tanks were among the Watch List tanks identified at the time.
The Ferrocyanide Hazard
Ferrocyanide is a complex of ferrous and cyanide ions that is stable in aqueous solutions. However, Sax's Dangerous Properties of Industrial Materials (3) states that "fusion of mixtures of metal cyanides with metal chlorates, perchlorates, nitrates, or nitrites may cause violent explosions." The hazard for the Hanford Site tanks was a sustainable, rapid exothermic reaction between ferrocyanide and nitrate/nitrite compounds also present in the waste. Sustainable means that the reaction can spread beyond a local ignition source and a rapid reaction is one that generates heat faster than it can dissipate. Excluded are slow, aging (degradation) reactions shown to occur over a period of years(4). A sustainable rapid exothermic reaction may produce sufficient heat and evolved gases to pressurize a waste tank headspace and release aerosols from tank vents and potentially damaging the tank's structure.
These chemicals can react violently if present in stoichiometric proportion, dry, and heated to temperatures above 250°C. However, information obtained over the past six years shows that ferrocyanide waste is composed mainly of inert chemicals and has a high water content; thus, the waste cannot support a self-sustaining reaction and cannot undergo the explosion scenarios postulated earlier. Conservative testing of ferrocyanide waste simulants has shown no evidence of explosive waste behavior. However, one simulant (In-Farm) will support a propagating reaction under special conditions. Laboratory studies of ferrocyanide waste simulants were used to determine conditions that would cause reactions and also conditions for which such reactions are not possible(5). Required conditions for rapid, sustained exothermic reactions are sufficient fuel concentration, sufficient oxidizer concentration (an excess is usually present in the tanks), relatively dry material, and a point-source initiator that can heat a portion of the material above the ignition temperature.
FERROCYANIDE WASTE ADDED TO THE TANKS
Whether an exothermic reaction in stored waste can occur depends on the relative concentrations of the reactants, inert solid diluents, water, and potential initiators that might be present. Historical records and knowledge gained from waste simulants produced from ferrocyanide scavenging process flowsheets was used to predict the major constituents and general physical properties of the ferrocyanide waste matrix.
The ferrocyanide scavenging campaign at Hanford was well documented, and most records were available(6,7,8). Amodel was developed from historical records to determine the inventory, concentration, and amount of ferrocyanide sludge originally placed in each ferrocyanide tank (9). The waste volumes are based on plant records and observed properties of flowsheet simulants. Three main scavenging flowsheets and variations thereof were used during the 1950s. These flowsheets were assigned the following values after validation tests(10):
Water concentrations (wt%) were calculated by centrifuging each flowsheet simulant and drying them in an oven at 105°C for 24 hours or under vacuum at 60°C. Surprisingly, it was found that the solids contained large quantities of water, even after centrifugation, as listed below:
The model does not account for degradation(4) of ferrocyanide over the 40 years of storage, nor does it account for dilution by mixing with other wastes added to the tanks after the scavenging campaign was completed. For these reasons, the model overpredicted actual ferrocyanide concentrations put into the tanks. Flowsheet data and ferrocyanide concentrations on the 18 ferrocyanide tanks are shown in Table I.
Table I Comparison of Tank Contents with Tank Safety Criteria

Reaction Energy
The most energetic reaction for oxidation of ferrocyanide is
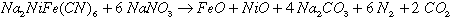 (1)
(1)
The theoretical exothermic energy ( H) for
this reaction is -9.5 megajoules per kilogram (MJ/kg) of ferrocyanide at 25°C
(298°K). Theoretical energy calculations show that nitrogen oxides are
formed in slow, lower temperature reactions between ferrocyanide and
nitrate-nitrite. For this case energies are much lower, or -6.6MJ/kg when N2O
is the product. If NO is produced, the reaction becomes endothermic at 0.73
MJ/kg. This wide variation in reaction energies illustrates the need for
experimental measurements to quantify the true energy available.
H) for
this reaction is -9.5 megajoules per kilogram (MJ/kg) of ferrocyanide at 25°C
(298°K). Theoretical energy calculations show that nitrogen oxides are
formed in slow, lower temperature reactions between ferrocyanide and
nitrate-nitrite. For this case energies are much lower, or -6.6MJ/kg when N2O
is the product. If NO is produced, the reaction becomes endothermic at 0.73
MJ/kg. This wide variation in reaction energies illustrates the need for
experimental measurements to quantify the true energy available.
Based on the results of three independent experimental studies on ferrocyanide waste simulants, a value of -6 MJ/kg of Na2NiFe(CN)6 was assigned for the reaction products produced(11). This is 63% of the theoretical energy for Eq. (1) and 90% of the energy if N2O is the product gas.
Calorimetry Tests. Calorimetry tests using 10 g and 70 g samples of ferrocyanide and NaNO3/NaNO2 mixtures as well as the flowsheet waste simulants were conducted at Fauske and Associates, Inc.(5). As the sample was heated to the reaction onset temperature and above, the thermal energy produced by the reaction causes the sample to self-heat. The rate and extent of this self-heating provides direct evidence of the character of the reaction that has taken place. The Fauske calorimeter cannot measure the effect of water on reactivity since the calorimeter heats the sample to dryness* before reaching the ignition temperature. These tests showed that all samples containing 15.6 wt% Na2NiFe(CN)6 or more can exhibit a propagating reaction while those with 12 wt% or less cannot. Additionally, it was found using a tube propagation apparatus(5) that propagating reactions even for ferrocyanide-rich mixtures are inhibited by small amounts of water.
Tube Propagation Tests. A special test apparatus for measuring the propagation velocity of the ferrocyanide reaction was also operated at Fauske and Associates. The reaction was ignited by a BaO2-Al mixture or a special electrical match with a given value of energy (typically 130J released in about 3 msec). The propagation of the reaction, if one occurs, is monitored by four thermocouples spaced 20 to 30 mm apart. Sharp temperature rises are noted as the reaction front passes each thermocouple. A reaction that continues to the end of the sample is capable of supporting a propagation reaction. If the material is not ignited or if it stops after only a short distance, then the sample cannot support a propagating reaction.
Using the calorimeter and the tube propagation apparatus, no propagating reactions were observed for sodium nickel ferrocyanide concentrations below 15 wt% on a dry (zero free water) basis (0.9 MJ/kg of waste). This is approximately double the 8wt% number derived using the theoretical energy. The 2:1 ratio of experiment and theory illustrates the degree to which the conservative theory underpredicts the experimentally observed ferrocyanide concentration required for a propagating reaction. With 25.5 wt% ferrocyanide (In-Farm-1 sludge, the most concentrated of the flowsheet simulants), propagation was observed with 8 wt% free water but not with 12 wt% free water. The 8 wt% Na2NiFe(CN)6 on a zero free water basis was chosen as the upper limit for safe mixtures.
These tests showed that propagation did not proceed or ceased when the free water content was 12 wt% or more. This applied to the simulant containing the highest concentration of ferrocyanide (25.5 wt%). The 12 wt% moisture threshold determined experimentally is less than half (only 43%) of the threshold indicated by the thermodynamic calculations. The difference is to be expected since thermodynamic calculations are inherently conservative. The simulants with concentrations high enough to propagate had burn velocities of less than 15 cm/minute.
Waste Safety Categories
The potential for an exothermic ferrocyanide reaction in the tanks was analyzed using the following: historical records that provided the tanks and flowsheet used to fill the tanks, calorimetry and propagation experiments performed with waste simulants, and analyses of chemical and physical processes that might intensify or diminish the ferrocyanide hazard. The necessary and sufficient conditions required for a ferrocyanide accident were determined via theoretical analysis and experiments. The results were then used to define conservative safety criteria for SAFE, CONDITIONALLY SAFE and UNSAFE waste storage. The values were selected to provide a safety margin between safe tank conditions and conditions where significant reactions could occur. The approved criteria are:
SAFE
- Concentration of fuel 8 wt% sodium nickel ferrocyanide
- Concentration of water - not limiting
- Concentration of oxidizers - not limiting
- Temperature of waste - not limiting.
CONDITIONALLY SAFE
- Concentration of fuel > 8 wt% sodium nickel ferrocyanide
- Concentration of water 0 to 24 wt%
- Concentration of oxidizers - not limiting
- Temperature of waste 90°C.
UNSAFE
- Criteria for SAFE and CONDITIONALLY SAFE are not met.
For tanks to be classified as SAFE, a low fuel content must be assured. For CONDITIONALLY SAFE tanks, retained moisture and maximum waste temperature must be assured so that they meet the safety criteria specified above. For tanks assigned to the UNSAFE category, it would be necessary to provide monitoring and controls sufficient to avoid conditions that could lead to reaction initiation.
Ferrocyanide Degradation by Aging
Aging of ferrocyanide waste is defined as thermal or radiolytic degradation of the ferrocyanide anion. This process breaks down the triple bond joining the carbon and nitrogen molecules and changes it into non-propagating compounds(4). Insoluble sodium nickel ferrocyanide, the major component of ferrocyanide sludges formed during the scavenging campaign, will dissolve in a caustic solution containing 0.01M or higher hydroxide ion. Dissolution of sodium nickel ferrocyanide results in soluble sodium ferrocyanide and a nickel hydroxide precipitate as shown in Eq. 2.
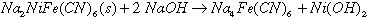 (2)
(2)
Under tank waste conditions, the ferrocyanide complex will hydrolyze to form formate, ferric oxide, and ammonia as follows.
 (3)
(3)
Recent experiments at the Pacific Northwest National Laboratory (PNNL) on ferrocyanide waste simulants(4) and available literature (as cited in Lilga et al. (4)) indicate that aging rates are strongly affected by temperature, radiation dose rate, and pH. Temperature has the greatest effect, but the rate of aging is also accelerated by a radiation field.
PNNL has investigated aging of ferrocyanide waste simulants for the last four years. They conducted experiments at 50, 70, 80, 90, and 100°C in 2 M NaOH solutions. Solutions were exposed to 60°Co gamma radiation at a dose rate of about 1 x 103 Sieverts per hour (Sv/h). Control solutions were also prepared and treated identically, except for the radiation exposure. Concentrations of ammonia, formate ion, and the other species produced via ferrocyanide ion hydrolysis were measured at various times.
Results showed that the ammonia concentration at each temperature increased almost linearly with time, and that the rate of hydrolysis increased with temperature.
Historical records for the 18 ferrocyanide tanks indicate that all of the tanks except the three tanks in TY Farm and TX-118 were operated at temperatures as high as or higher than 75°C. The BY Farm tanks, where the majority of the ferrocyanide waste was stored, were part of the "In Tank Solidification" program conducted from 1966 to 1976. Waste was purposely heated in tanks BY-101, BY-102, and BY-112 to evaporate excess water. The waste was heated to high temperatures, up to 125°C, and subsequently pumped to the other 9 BY tanks for cooling. As the waste cooled the part of the enriched supernatant crystallized as saltcake. Based on PNNL aging experiments, the BY-Farm temperatures were high enough to degrade the concentration of ferrocyanide to about zero in just a few years. Four BY tanks were sampled and all showed non-detectable or very low concentrations of cyanide. On the other hand, significant levels of nickel were measured thus indicating that the ferrocyanide was present at one time. Temperatures in BY-108 were the lowest of the ferrocyanide tanks in that farm, but this was one of the four tanks sampled. It is the bounding tank for the BY Farm. Based on BY-108 results, the BY ferrocyanide tanks were categorized as SAFE.
Temperatures in the three TY Farm ferrocyanide tanks probably did not exceed 55°C, although there is an operating record showing that tank TY-104 received 100+C waste in 1969-70. Only tank TY-104 was sampled; results show that an insignificant amount of cyanide remains in that tank. Because temperatures in TY-104 were lower or about the same as TY-101 and TY-104, and all three of the TY tanks received the same ferrocyanide flowsheet waste, it was concluded that the ferrocyanide in TY-101 and TY-103 also degraded and these tanks were categorized as SAFE.
Actual temperature data for tank TX-118 were not found for the 1960s and 1970s; however, transfer records show that this tank should not have been put on the list of ferrocyanide tanks in 1989(12). The records do not show any transfer to this tank that contained ferrocyanide solids; however, it was included because it was erroneously identified as containing ferrocyanide in the Tracking Radioactive Components (TRAC) document(13). Another record(14) shows evaporator bottoms were received from the 242- T evaporator from 1969 until 1977. Evaporator waste would have been high enough in temperature to degrade any ferrocyanide present. Hanlon(15) shows that the tank contains all saltcake with no sludge. Based on the above records, the tank was also categorized as SAFE.
The C-Farm tanks (C-108, C-109, C-111, and C-112) received waste with the richest amount of ferrocyanide, from the In-Farm flowsheet. Simulants produced by this flowsheet were the only ones that would propagate when dry. At least three of these tanks experienced high enough temperatures to enhance ferrocyanide degradation, with temperatures recorded during the 1960s in the mid-70°C to the high 80°C range. No temperature data were found for C-108 back to the 1960s when these tanks were warmer. However, a review of the operating history indicates that C-108 should have experienced temperatures similar to C-109. The temperatures in these tanks were high enough to enhance ferrocyanide aging. All four of these tanks were sampled and all show degradation of ferrocyanide by a factor of 10 or more; they were also categorized as SAFE.
The remaining ferrocyanide tank, T-107, was sampled and results show insignificant amounts of total cyanide; this tank too is categorized as SAFE.
The PNNL temperature experiments on ferrocyanide waste simulants described above(4) also show that gamma radiation accelerates ferrocyanide aging. Non-irradiated simulants aged up to one order of magnitude less than irradiated samples under similar conditions of time, pH, and temperature.
Gamma dose rate experiments to determine the rate of hydrolysis of ferrocyanide simulants were conducted at 90°C in 2 M NaOH solutions. Ammonia production was again essentially linear, increasing with increasing dose rate. The high ferrocyanide concentration C-Farm tank waste has been exposed to date to a relatively high integrated gamma dose (between 2.4x 106 to 5.3 x 106 Sv. The maximum integrated dose in the PNNL aging experiments was about 5x 105 Sv, an order of magnitude less than that experienced by the C-Farm tank waste. Based on these experiments, the radiation exposure in the tanks was more than sufficient to significantly age the ferrocyanide waste.
Ferrocyanide waste aging by dissolution and then hydrolysis (Eqs. 2 and 3) was most effective under highly alkaline conditions, because ferrocyanide becomes soluble from dissolution of sodium nickel ferrocyanide at high pH. However, experiments were also conducted at pH 10, where sodium nickel ferrocyanide has low solubility. Results from these tests show that hydrolysis still occurs in the solid phase, but at slower rates(4).
The ferrocyanide precipitation processes were done at slightly alkaline
conditions (pH  9-10). Later, a variety of waste
operations introduced highly alkaline waste into the tanks(14). Samples from the
11 ferrocyanide tanks sampled had pH values above 10 and many samples showed a
pH > 12. The BY-Farm tanks, in particular, received highly alkaline saltcake
waste.
9-10). Later, a variety of waste
operations introduced highly alkaline waste into the tanks(14). Samples from the
11 ferrocyanide tanks sampled had pH values above 10 and many samples showed a
pH > 12. The BY-Farm tanks, in particular, received highly alkaline saltcake
waste.
Historical operating records show that all the ferrocyanide tanks contained, subsequent to the ferrocyanide campaigns, enough caustic to enhance aging, some tanks more than others. Consequently, waste pH was not a limiting factor for ferrocyanide waste aging in Hanford Site tanks.
Based on tank histories and laboratory experiments with simulated ferrocyanide flowsheet materials, conditions existed within the tanks over the past 40 years that greatly promoted ferrocyanide aging. Caustic solutions, high temperature, and the presence of gamma radiation all contribute to hydrolysis of the ferrocyanide ion. The experimental results are consistent with the analytical results from actual tank samples. The latter show the expected amount of nickel but very low cyanide and energy content, thus proving that aging ferrocyanide ion occurred in the waste tanks.
WASTE SAMPLE RESULTS
Nine ferrocyanide tanks (BY-104, BY-108, BY-110, C-108, C-109, C-111, C-112, T-107, and TY-104) were sampled that met the sampling requirements specified in the Ferrocyanide Data Quality Objectives (DQO) document(16). A tenth tank (BY-106) was also sampled but only a few grams of sludge were obtained from one riser. The rest of the samples were all saltcake where no ferrocyanide was detected. The amount of ferrocyanide measured for the tank samples is shown in column 5 of Table I.
Aging Confirmed
To confirm that aging of the ferrocyanide actually occurred in the waste, the Ferrocyanide DQO(16) provided for historical data and aging models to be corroborated using measurements of fuel and nickel concentrations in the waste. To determine if aging had occurred, the fuel concentration was measured first. If the fuel concentration was less than predicted by the process flowsheets, then aging may have been the reason. Next, the measured nickel concentration was used to confirm or reject historical information and ferrocyanide aging models. Since the nickel added as part of the ferrocyanide scavenging flowsheet was the only source of high nickel concentrations added to the tanks, it could be used as a signature analyte to confirm that ferrocyanide was once present.
Waste simulants produced using the original flowsheets(17) showed nickel concentrations ranging between 0.87 to 4.8 wt% on a dry-weight basis. A lower bound of 0.8 wt% or 8,000 µg/g (dry-weight sample basis) was selected as a minimum value needed to confirm that the tank originally contained ferrocyanide sludge.
If the waste samples had sufficiently high nickel concentrations to conclude that it originally contained ferrocyanide sludge, then waste aging was confirmed. If the waste had a low nickel concentration, then the tank might have been erroneously identified as containing ferrocyanide waste, or another explanation may apply. For example, many of the ferrocyanide tanks received saltcake waste that showed little or no nickel present, while other ferrocyanide tanks received just sludges. None of the added waste would have contained nickel. Additional mixing that undoubtedly occurred under some of the risers would have diluted the nickel results.
Aging in the Worst-Case Tanks
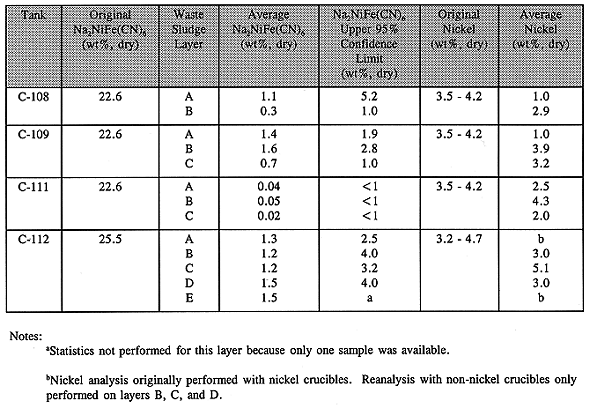
As indicated above the four C-Farm tanks (C-108, C-109, C-111, and C-112) received the richest concentration of ferrocyanide via the In-Farm flowsheet. This flowsheet used only the supernatant and not the waste solids as in the case of the U-Plant and T-Plant flowsheets. As a variation of the In-Farm flowsheet, tank C-112 received the highest concentration (see Table I). As stated earlier waste produced by the In-Farm flowsheet did propagate when dry and heated to a temperatures above 250°C; hence these four tanks became an early focus of concern. The first tanks to be sampled were C-112 and C-109.
Analyte concentrations determined from the tank samples are listed in Table II along with the original concentrations calculated from the process flowsheets and determined experimentally(10). These data were treated statistically to provide an upper 95% confidence limit. Total cyanide, energetics, and nickel analyses of the waste samples confirm that the ferrocyanide in these tanks has aged. As seen in the table, the average ferrocyanide concentrations were at least a factor of ten less than the original concentrations. Because an insufficient amount of fuel is present in these tanks, they are categorized as SAFE.
Table II Current Ferrocyanide and Nickel Concentrations Compared to
Original Concentrations (Dry Basis) for C-Farm Tanks
CONCLUSIONS
The U-Plant and T-Plant ferrocyanide flowsheet wastes added to the 14 Hanford Site tanks constitutes about 74% of the ferrocyanide added. This fraction of waste was insufficient in fuel value when first formed to support a propagating reaction even if dry (zero free water). However, the remaining fraction of ferrocyanide waste produced by the In-Farm flowsheet was significantly higher in ferrocyanide concentration and was stored in four C-Farm tanks. Samples were obtained and analyzed from ten of the ferrocyanide tanks including all four of the worst-case C-Farm tanks. These tanks bound the aging conditions for the unsampled tanks. The results showed that all the waste in the 18 ferrocyanide tanks are now too low in ferrocyanide concentration to support a propagating reaction. This decrease in concentration is credited to chemical aging that occurred over the 40 or more years of storage. The tanks are now categorized as SAFE with regard to the Ferrocyanide Safety Issue.
The ferrocyanide aging studies and verification that aging occurred in all of waste samples from ten ferrocyanide tanks led the U.S. Department of Energy in September 1997 to remove all 18 ferrocyanide tanks from the Watch List. Ferrocyanide propagating reaction accidents are concluded to be beyond extremely unlikely events. The DNFSB closed their Recommendation 90-7 in September 1997 and concurred with DOE in December 1997 that the Ferrocyanide Safety Issue is now resolved. The technical document that supports these decisions is WHC-SD-WM-SARR-038, Rev. 118.
*The ferrocyanide simulants contained both bound (hydrated) and free water. Free water can be removed using standard drying methods. Na2NiFe(CN)6 contained approximately 4.6 moles of bound water that was not released until the sample approaches 160°C and higher.
REFERENCES