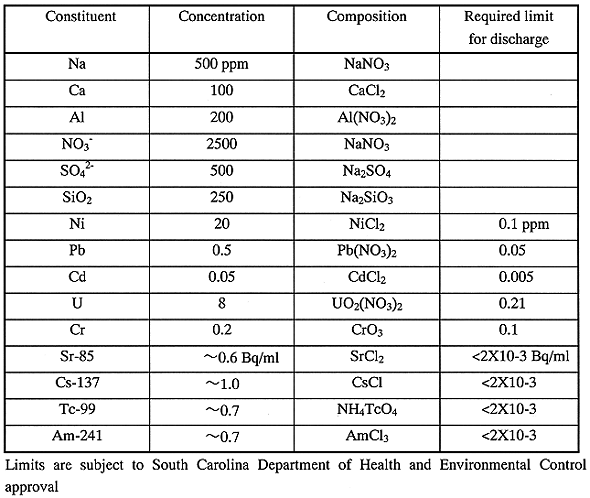
Table I Chemical Composition of Simulant
K. Suzuki and M. Hirano
Oarai Nuclear Research Center
JGC Corporation
2205 Naritacho Oaraimachi
Ibaraki 311-13 Japan
81-29-266-3311
T. Nakashima, Richard L. Baker and Phil N. Baldwin
ADTECHS
2411 Dulles Corner Park Suite 520
Herndon VA 22071
703-713-9000
ABSTRACT
This study investigated the performance of coagulation for treatment of ground water containing Cd, Cr, Ni, Pb, U and radionuclides. Additive reagents of TMT, Na2HPO4, and an emulsion breaker were satisfactorily used to remove heavy metals and Am radionuclide. It was shown that the selective adsorbent, zeolite, has an effective ability to completely eliminate radioactivity of Cs and Sr, and that Dowex anion resin adsorbs Tc-99 selectively. A basic process consisting of coagulation and selective adsorption was discussed.
INTRODUCTION
Ground water contaminated with constituents of RCRA metals and radionuclide metals in DOE sites should be properly treated to protect environmental safety. Cd, Cr, Ni, Pb, U, Cs-137, Sr-90, Tc-99, etc. should be removed and stabilized (1).
ADTECHS and JGC Corporation studied a basic process consisting of reverse osmosis (RO) and coagulation for the treatment of ground water. In the first step of the process, ground water is treated by RO in order to recover the purified water. The liquid waste concentrated by the RO process is coagulated to form insoluble precipitates of heavy metals, U and radionuclides.
This paper reports and discusses chemical behavior of the coagulation and performance of specified adsorbents to effectively remove Cs-137, Sr-90 and Tc-99.
EXPERIMENTAL METHOD
A simulated liquid waste corresponding to the concentrate of ground water by RO was prepared with chemical compositions shown in Table I. Concentration of constituents was selected, based on the example of DOE site in South Carolina. Radioactivity of radionuclides was about 100 times higher than the detectable limit to easily measure Decontamination Factor (DF) for removal of radionuclides. The required limit of each constituent for discharge in the table was a target value subjected to SCDHEC approval.
Table I Chemical Composition of Simulant

To investigate chemical reactions in the coagulation process, trisodium trimercapto-s-triazine,TMT(Degussa Corp. in USA) and sodium phosphate, Na2HPO4 were used as metal precipitating agent, respectively. In experiments, 15wt% TMT solution was prepared and the volume of TMT solution, 1.8ml, stoichiometrically needed to react with 100ml of the simulant. In the case of sodium phosphate, 1ml of 3.5wt% Na2HPO4 solution was added to 100ml of simulant, because Ca ion in the simulant stoichiometrically reacted with HPO42-. Moreover, emulsion breaker, SPILIT 300(Diversey Corp. in USA) was also used.
All experiments for the coagulation were carried out on bench scale apparatus at room temperature. Precipitates in the treated solution were separated by a membrane filter. The concentration of constituents in the filtrated solution samples was measured by ICP-MS (Inductively Coupled Plasma Source Mass Spectrometry(Yokogawa Electric Corp., PMS-200)). The detectable limit was checked with the use of a standard solution containing each metal.
Selective zeolite exchanger of Cs, SIR-600 (ResinTech Inc. in USA ), and anion exchange resin, DOWEX 21K (The Dow Chemical Company in USA) were selected and used in the adsorption study of Cs, Sr and Tc radionuclides.
RESULTS AND DISCUSSIONS
Addition of TMT in Coagulation
TMT was added to the simulant with an emulsion breaker in the range of pH 4 to pH 10.
Table II summarizes experimental results. Uranium, UO22+, is effectively stabilized at a neutral pH in the presence of TMT. Pb and Cd are insolubilized with increasing amounts of TMT at higher than pH 7. The residual concentrations of U, Pb and Cd in the treated solution were less than the required limit values shown in Table I. It is known from this experiment that heavy metals in simulant form effectively insoluble precipitates under the lower amount of TMT than stoichiometric addition.
On the other hand, the residual concentration of Ni in the filtrate decreases mainly with increased pH values, regardless of the addition of TMT. At higher pH values than pH 9, the residual concentration in the solution becomes less than the limit value. It is revealed that Ni in the simulant is properly insolubilized with pH increase rather than the addition of TMT.
An effect of SPILIT addition is shown in Fig. 1. It is difficult to separate the dispersed precipitate in the solution without SPILIT.
Table II Effect of TMT Addition on Coagulation
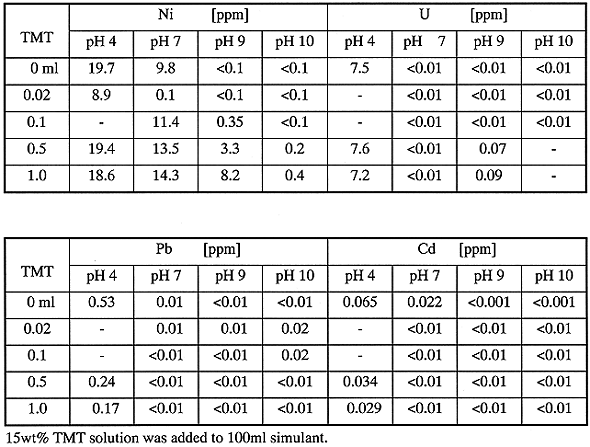
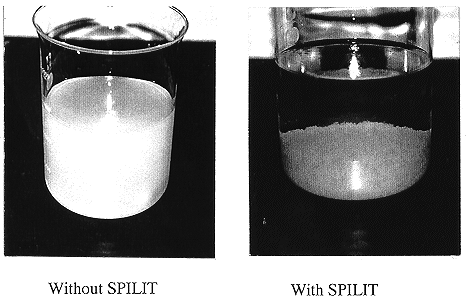
Fig. 1. Effect of emulsion breaker on
coagulation.
Coprecipitation of Radionuclides
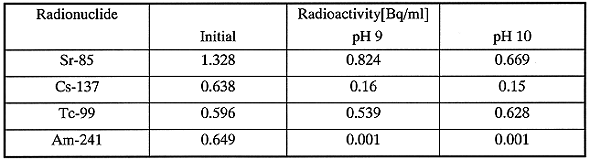
The removal of radionuclides from the simulant was studied at pH 9 and 10 in the presence of SPILIT. Table III shows the results. Am-241 is completely removed from the solution in the coagulation, and the residual radioactivity achieves less than the required limit. The residual radioactivity of Cs-137 and Sr-90 is about one-fifth and half of initial radioactivity, respectively. Because SPILIT contains a kind of clay material, the decrease of radioactivity is due to the effective absorbency of the clay for Cs and Sr.
Tc-99 is not removed in the coagulation, because the chemical state is anion which has more difficulty in forming an insoluble precipitate than metal cations.
Table III Coprecipitation of Radionuclides at pH9 and 10
Addition of Na2HPO4 in Coagulation
An addition of Na2HPO4 was considered in order to improve the removal of Ni, U and Srradionuclide in the simulant. This phosphate salt is suitable to produce insoluble precipitates of metal cations, because U, Sr and Ni are able to form insoluble phosphate salts.
Table IV shows the results. Sr radionuclide is effectively insolubilized and coprecipitated by a formation of calcium phosphate precipitate, because the simulant contains a large amount of Ca ion. The residual concentrations of both U and Ni ions are less than the required limit values, and uranium ion is effectively insolubilized, compared with the addition of TMT. It is indicated that the formation of insoluble precipitates of U, Ni and Sr radionuclide in the simulant is effectively achieved. by the addition of Na2HPO4
Table IV Coagulation of Simulant by Addition of Na2HPO4
Selective Adsorption of Cs Radionuclide
A selective adsorbent for Cs, zeolite, was used to treat the solution, because Cs was one of the residual radionuclides present in greater than the required limit in the solution treated by the coagulation. The selected adsorbent, SIR-600, contains mainly a natural clinoptilite, one of the natural zeolites, to adsorb Cs and Sr ion selectively.
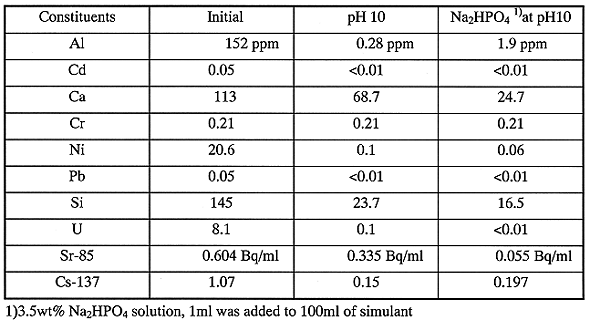
A small column filled with SIR-600 was used in the experiment. After the simulant was treated by the addition of both SPILIT and Na2HPO4 at pH 10, the solution in the coagulation was filtered. Then, the filtrate was through the column. Figure 2 illustrates a break-through curve of the adsorption. Even at a bed volume =1100, radioactivity of Cs-137 in the effluent from the column is less than the detectable limit. Bed volume means ratio of the treated solution volume to the filled adsorbent volume.
Sr radionuclide is also removed effectively with SIR-600 in these experimental conditions. It is confirmed that a combination of coagulation and selective adsorption implements the complete removal of Cs and Sr radionuclides.
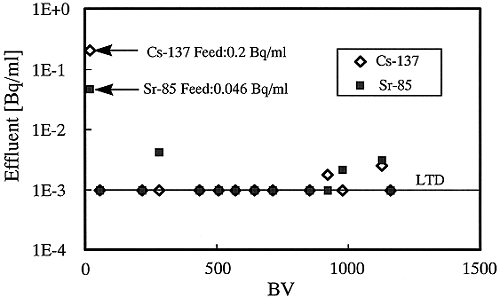
Fig. 2. Adsorption of Cs-137/Sr-85 by
Cs selective zeolite exchanger.
Removal of Tc by Adsorption
Chemical species of Tc-99 found in ground water would be in anion state, TcO4-. This anion was not removed, in both the coagulation and the Cs selective adsorption by SIR-600. In this study, selective adsorption with DOWEX 21K is used in order to remove Tc-99 from the simulant, because this absorbent is one of the anion resins giving strong absorption of Tc-99 (2).
Results are shown in Fig. 3. DOWEX 21K has a specified ability to remove Tc-99, and there is no break-through until the bed volume exceeds 1200. DF value in this adsorption is higher than DF=100.
Fig. 3. Adsorption of Tc-99 and
Cr(VI) by DOWEX 21K resin.
Treatment of Cr(VI)
The result of CrO4- adsorption is also illustrated in Fig. 3. The adsorption appears at the break-through of Cr(IV) at only BV = 270. All of the results mentioned above suggest that Cr(VI) would consequently be a residue metal ion in the final solution, which is directly treated by coagulation and adsorption of both SIR-600 and DOWEX 21K. To effectively remove Cr(VI), the metal ion in the simulant should be first reduced to Cr(III) by the use of a reductant, NaHSO3, because it is well known that Cr(III) easily forms a hydroxide precipitate at higher than pH 7. Table V indicates the results of coagulation of Cr(III). The concentration in the solution is less than the required limit value. The pretreatment of reduction is effective to remove Cr(IV) inthe solution.
Table V Reduction of Cr(VI) with NaHSO3 and Coagulation
at pH10

BASIC FLOW OF PROCESS
This study suggests that the performance of coagulation reaction is complicated and varies in the treatment of ground water contaminated with heavy metals and radionuclides. Then, two conditions are assumed in order to evaluate a basic process flow;
The basic process flow consists of coagulation and selective adsorption, schematically shown in Fig. 4. For Cr(VI) treatment, reduction with NaHSO3 should be carried out before the coagulation. In Case-1, the addition of TMT will be recommended in the coagulation process, because a small amount of TMT is able to effectively remove uranium, Pb, Cd and Cr(III) at a neutral pH. Also, the preparation of neutral pH is comparatively easy, because the solution concentrated by RO would be in weak acid pH ranges in general.
In Case-2, Na2HPO4 solution is used at pH 10 for the coagulation. Ni, U, Pb, Cd and Sr radionuclide will be effectively removed from the solution. Also, SPILIT emulsion breaker should be added to the coagulation solution to separate the dispersed precipitates. In order to eliminate radioactivity of Cs, Sr and Tc completely, the selective adsorption process is necessary in both Case-1 and Case-2. The Cs selective zeolite containing clinoptilite and DOWEX 21K plays an important role in the adsorption.
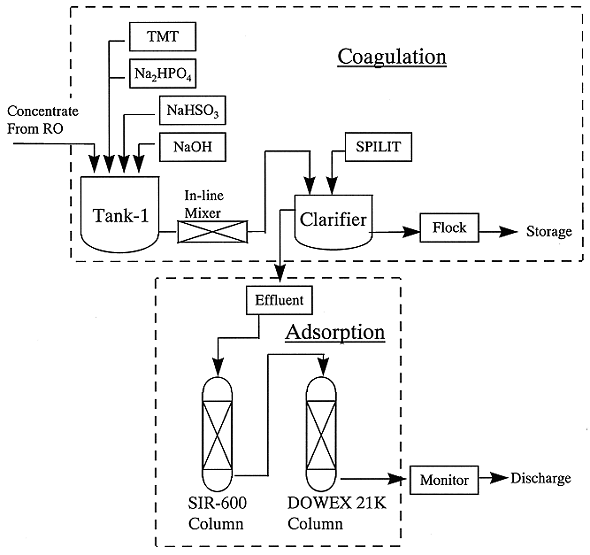
Fig. 4. Process flow of treatment for
removal of heavy metals and radionuclides in ground water.
CONCLUSIONS
The coagulation reaction was experimentally studied for the treatment of ground water containing U, Ni, Pb, Cd, Cr(VI) and radionuclides of Cs, Sr and Tc. The main conclusions are summarized;
REFERENCES