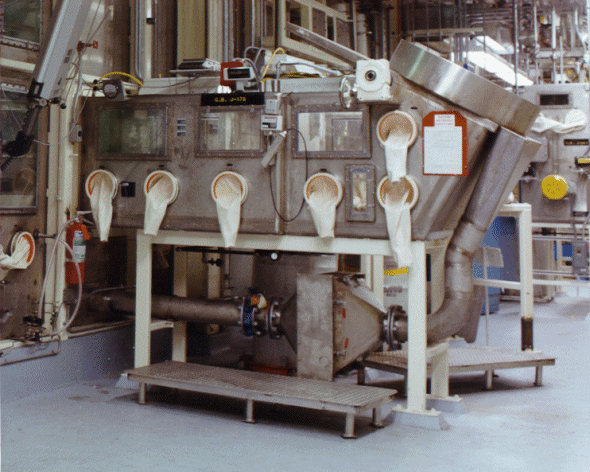
Fig. 1 Photograph of the advanced
size reduction facility (ASRF)
G.A. OLeary, P.M. Arnold and E.L. DAmico
Rocky Mountain
Remediation Services, L.L.C.
ABSTRACT
In the late 1960s problems were observed at Rocky Flats and Mound with nitrated leaded rubber gloves. Various reports documented the formation of a yellow crystalline compound of lead nitrate in a matrix of organic material (carboxylic acid). Recent studies conducted at the Rocky Flats Environmental Technology Site (RFETS) and the Colorado School of Mines also confirmed these earlier findings. In addition to the lead nitrates, these controlled analytical experiments looked for the presence of fulminates that were also thought to form. No fulminates were found in the reaction of the lead oxide inner glove layer with nitric acid. A yellow crystalline product was observed (consistent with the earlier studies) that, when separated, had a flammability temperature between 175 and 200 degrees centigrade. Infrared (IR) analysis of this material indicated the presence of short-chained carboxylic acids with randomly substituted nitro-groups, which was also consistent with the earlier studies.
The Rocky Mountain Remediation Services (RMRS) L.L.C developed a project to inspect and process containers of transuranic-mixed waste acid-contaminated leaded rubber gloves to eliminate any potential risk associated with the formation of the lead nitrate. This process utilized existing facilities to process the gloves and the byproduct rinse water. RMRS is the waste management subcontractor at RFETS, managing waste generated by all site subcontractors. In this capacity RMRS is responsible for storing, treating and disposing of all waste generated at the site.
INTRODUCTION
The Rocky Mountain Remediation Services L.L.C developed a project to process 55-gallon drums of transuranic-mixed waste acid-contaminated leaded rubber gloves. The process included: a) installation of a carbon composite filter on the 55-gallon drumlid and venting the 90 mil rigid poly liner lid, and b) inspecting, rinsing, and repackaging each individual acid-contaminated leaded rubber glove. These processes were required to eliminate any potential risk associated with the accumulation of explosive gases in the headspace of the drum and the formation of lead nitrate on the leaded gloves.
BACKGROUND
In the late 1960s, problems were observed at Rocky Flats and Mound with nitrated leaded rubber gloves. Reports (1, 2, 3) documented the formation of a yellow, crystalline compound of lead nitrate in a matrix of organic material (carboxylic acids). The yellow compound burned intensely at elevated temperature and was reported to be shock sensitive. The nitric acid reacted with the inner lead oxide layer of the glove through cracks, abrasions or diffusion to produce the product. It was demonstrated that water washing of the gloves removed any reaction product and a glove-washing process was used at Rocky Flats from the early 1970s until approximately 1987.
Also in approximately 1987, a new Item Description Code (IDC) was initiated to track the leaded rubber gloves generated in gloveboxes using nitric acid: IDC 341, Leaded Rubber Gloves, Acid-Contaminated. The original IDC 339 was clarified to identify leaded rubber gloves, not acid-contaminated and represented approximately one-half of gloves generated. From 1987 to late 1989, when site reprocessing operations were suspended, drums potentially containing nitrated lead compounds were likely to have been generated. Therefore, all drums of IDC 341, Leaded Rubber Gloves, Acid-Contaminated, needed to be processed to inspect and remove any reactive compounds.
DISCUSSION
As stated in the introduction, the acid-contaminated leaded rubber glove project included several activities. This project was established to be conducted in two phases. Phase I, which was completed on May 16, 1994, primarily focused on installing drum lids fitted with carbon composite filters and performing controlled analytical experiments to identify and characterize the products formed when leaded rubber gloves are exposed to nitric acid. Phase II focused on inspecting, rinsing, and repackaging of acid-contaminated leaded rubber gloves, IDC 341 utilizing an existing facility at RFETS. The sampling activities were limited to sampling the liquid waste generated by the rinsing operations.
Phase I
In Phase I of the acid-contaminated leaded rubber glove project, all unvented drums of acid-contaminated leaded rubber gloves were fitted with lids equipped with nuclear grade carbon composite filters and the 90 mil rigid polyethylene liner was punctured to allow accumulated gases to escape. The generation of hydrogen gas from hydrogenous material in contact with radionuclides is well documented. Therefore, the primary objective of this phase of the project was to remove the potential of hydrogen explosion serving as an initiator for reactive compounds on the gloves while studies on reaction products were completed. RFETS has developed a system for the safe venting, and sampling if necessary, of potentially flammable or explosive drum atmospheres. In addition to the drums vented under this project, the system has been used to safely collect over 900 drum or inner bag headspace gas samples and to vent over 4,400 drums, thereby significantly reducing risk from the stored waste inventory.
Prior to performing the venting operation, the site operating system database was utilized to search for unfiltered 55-gallon drums of acid-contaminated leaded rubber gloves - 67 unfiltered drums were found. Also physical verification was conducted on other leaded rubber glove drums to ensure that they were filtered. Following identification and location of the unfiltered drum inventory, management approval was obtained to move the 55-gallon drums within buildings, and install filtered drum lids and puncture the 90 mil rigid polyethylene liner. This work was conducted under a strict conduct of operations regime due to the potential risks involved.
An additional part of this phase consisted of controlled laboratory experiments conducted to identify and characterize the products formed when leaded rubber gloves (lead-oxide sandwiched between neoprene and Hypalon®) were exposed to various nitric acid concentrations at differing temperature and exposure time durations. One set of experiments was conducted onsite and the second was conducted at the Colorado School of Mines. The underlying purpose of the experimental and analytical work was to determine if a safety hazard existed as a result of leaded gloves being exposed to nitric acid by analyzing for the formation of shock-sensitive compounds, including lead fulminate, Pb(OCN)2.
The on-site study indicated that no fulminates were found in the reaction of the lead oxide inner glove layer with nitric acid. A yellow crystalline product was observed that, when separated and dried, had a flammability temperature of approximately 200°C. Infrared (IR) analysis indicated that this material showed the presence of short-chained carboxylic acids with randomly substituted nitro- groups, which was consistent with previous findings.
An additional study at the Colorado School of Mines was conducted to characterize the reaction products as a result of exposing the leaded gloves to nitric acid and to determine the flammability and shock sensitivity of the reaction products (4). This study indicated that the outer Hypalon® layer of the leaded rubber gloves was practically unaffected by the acid, but the neoprene and lead oxide components react with nitric acid to produce a yellow compound that has a flammability temperature of 175°C. The reaction occurs at room temperature with 12M acid while elevated temperatures are required for lower concentrations. Analysis by infrared and x-ray diffraction determined that no fulminates were present. The reaction product was a mixture of lead nitrate and an unidentified organic compound. The study concluded that washing of used gloves in water can eliminate the possibility of any explosion that might occur with the product due to an increase in temperature.
Phase II
In Phase II of the acid-contaminated leaded rubber glove project, inspecting, sampling, washing, and repackaging activities were developed and implemented in the Barrel Dump Glovebox of the Advanced Size Reduction Facility (ASRF). Figure 1 shows a photograph of the ASRF, including the location of the Barrel Dump Glovebox.

Fig. 1 Photograph of the advanced
size reduction facility (ASRF)
Once the waste was introduced into the Barrel Dump Glovebox, the contents were inspected for indications of nitric acid contamination or degradation, and against the criteria established on an information checklist for drums containing leaded rubber gloves. The inspection criteria included indications of any cracks, blisters, softening, yellow product, or any other indication of nitric acid degradation of the lead oxide layer. After inspection, the gloves were sorted into two categories: non-acid reacted and acid reacted gloves. The non-acid reacted gloves were wiped with a wet wash cloth. The acid reacted gloves were washed with warm water. Upon completion of the rinsing operation the leaded gloves were dried and repackaged into a 55-gallon drum. Each 55-gallon drum of the processed leaded gloves was properly labeled and transferred for nondestructive radioassay before being placed back into storage. The process flow for performing this operation is shown in Figure 2.
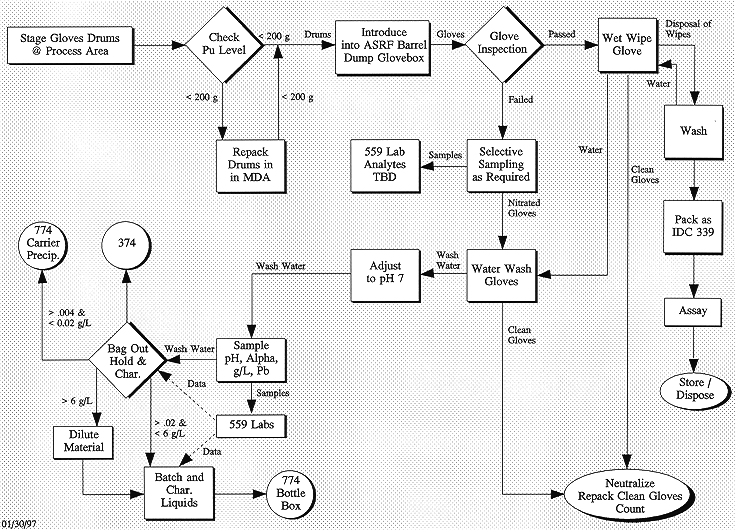
Fig. 2. Process flow diagram of the
leaded rubber glove washing process.
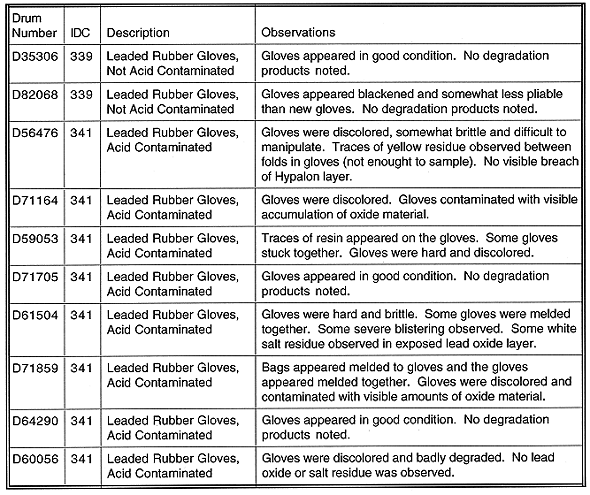
The observations of the leaded gloves inspected from 10 drums indicated various degrees of deterioration on the gloves and the inner packaging due to nitric acid exposure. Visual observations for the packaging materials included discoloration of the inner-most poly bag used to contain the leaded gloves, discoloration and degradation of the tape used to seal the poly bags, and the poly bags appearing burnt and melded to the gloves. No significant deterioration of the outer packaging materials such as the drum itself and the rigid liner was observed.
In several instances, the leaded gloves themselves appeared discolored and darkened, melded together as well as hard or brittle. Some plutonium oxide was observed on the leaded gloves and in some cases traces of yellow salt or residue-like material were observed as well. There was an insufficient amount of the yellow salt or residue to sample effectively, therefore, no samples were collected. A summary of the observations is found in Table I.
Table I Summarized Observations on Physical Condition of Leaded
Rubber Gloves
The wash water obtained from the rinsing process was sampled and analyzed. The pH of the wash solution was on the order of 3 to 4. The concentration of lead in the wash water varied from 13 to 18 parts per million while the plutonium concentration was on the order of 2 grams per liter.
The liquids generated from the rinsing process will be immobilized by an existing onsite cementation process. This process known as the Bottlebox is used to immobilize miscellaneous aqueous waste solutions. This treatment process will render the solutions from the leaded glove rinsing operation non-corrosive, complex Land Disposal Restricted (LDR) constituents, and solidify the waste solutions for disposal.
CONCLUSION
The RFETS has a legacy of in-process waste forms that are a primary result of curtailment of operations in 1989 and the change in mission in 1992. One of these waste streams, leaded rubber gloves used in gloveboxes containing nitric acid, was identified as a high priority stream requiring action to mitigate potential risks. Therefore, the RMRS Waste Management organization first vented all drums to eliminate the potential for hydrogen explosion to serve as an initiator for any heat-sensitive compounds in the leaded gloves. Following venting, washing operations were conducted on the gloves to remove any potential reactive compounds and make the waste stream ready for final disposal.
This project was completed through committed cooperation among the subcontractors and the integrating management contractor at RFETS under conduct of operations work practices. Venting was performed using processes developed for safely processing transuranic waste drums. Existing gloveboxes and equipment was used in conjunction with newly developed procedures to conduct the glove washing work in the most cost effective manner to reduce the risks from this waste stream. Although the chemical reactions that can occur on these gloves present a significant potential risk, based on the actual observations there was little identified reactive material on the gloves.
REFERENCES