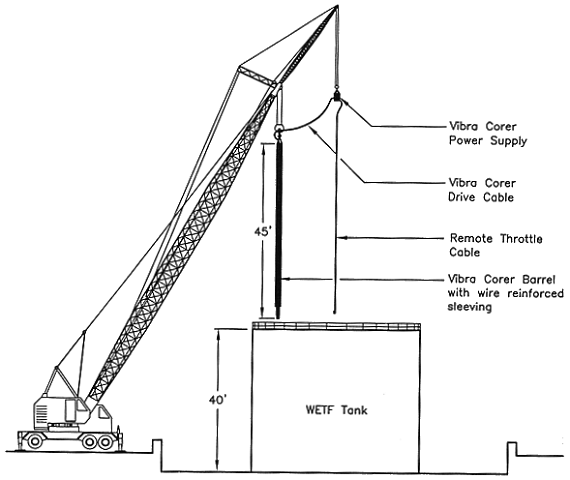
Fig. 1. Vibrating corer extraction
setup.
Matt Cage
Scientific Ecology Group
ABSTRACT
Between 1994 and 1996, the Scientific Ecology Group, Inc. (SEG), supported by Envitco, Inc., performed a proof-of-process demonstration test for vitrification of West End Treatment Facility (WETF) mixed waste sludge generated at Oak Ridge's Y-12 Plant. The test was the largest mixed waste vitrification demonstration to date; 7011 lb. of surrogate and 6815 lb. of dried WETF feed were successfully vitrified, producing respectively 5008 and 4362 lb. of stable glass product with a projected full scale volume reduction (VR) ranging from 2.8:1 to 13.8:1, depending on the percent solids in the sludge. The vitrification unit used in the demonstration test was the Envitco Waste-Vit® EV-101, a two ton/day joule-heated melter which operates at temperatures up to 2700°F. As the WETF sludge is a mixed waste, the objective for the treatment process was for the glass waste form to meet both the RCRA Land Disposal Restrictions (LDR) and the disposal site waste acceptance criteria (WAC).
Bench tests were conducted to select glass forming ingredients that produced a processable glass that maximized waste loading without negatively impacting life-cycle costs through either increased wear on melter refractory or increased energy requirements. In the proof of process demonstration, three melter volumes of surrogate were processed to achieve steady state vitrification conditions. The waste surrogate was chemically similar to the actual WETF waste and included a lead and cadmium spike to demonstrate the robustness of the treatment process. Actual mixed waste glass produced met the proof-of-process objectives by meeting the disposal site WAC and RCRA LDR with 95% confidence.
The system proved highly reliable with a 95% availability during the two week test. The melter was operated at 40% of the design capacity, which is based on the melting rate for dry batch soda-lime-silica (SLS) glass. From the demonstration, several process improvements have been identified to increase the throughput to near SLS capacity, including:
INTRODUCTION
Under the Federal Facilities Compliance Act (FFCA), Department of Energy (DOE) sites in the United States are required by the Environmental Protection Agency (EPA) to treat and dispose of their mixed waste inventories by established schedules. The top priority mixed waste stream designated for treatment under the FFCA in the State of Tennessee is the tank sludge generated from the West End Treatment Facility (WETF) at Oak Ridge's Y-12 Plant. To comply with the FFCA and support the DOE privatization initiatives, Lockheed Martin Energy Systems (LMES), the operator of the Y-12 Plant, issued a contract to Scientific Ecology Group (SEG), with Envitco as a vitrification subcontractor, to perform a proof-of-process demonstration test for vitrification of the WETF sludge. LMES used a multi-phased approach to mitigate risk, where the Phase I demonstration test, which is summarized in this report, was to be followed by Phase II full-scale treatment.
To accomplish the Phase I demonstration, SEG extracted over 8000 lb. of sludge by core sampling the four WETF tanks at the WETF tank farm. The sludge was transported to the SEG Oak Ridge facility to conduct bench scale tests and the proof of process demonstration. In the bench scale tests, a variety of glass formulations were tested to maximize waste loading while yielding a processable glass with suitable viscosity and minimal refractory corrosion potential. The optimum formula from the bench scale tests was used for the demonstration test, where the waste was vitrified in a full scale two-ton/day Envitco EV-101 Waste-Vit® melter.
The objective for the proof-of-process test was to demonstrate that the entire inventory of the WETF sludge could be processed while producing a glass waste form (and ancillary waste forms) that meets the RCRA Land Disposal Restrictions (LDR) and the disposal site waste acceptance criteria (WAC). The demonstration objectives were met in the performance of the largest mixed waste vitrification demonstration to-date; 7,011 lb. of surrogate and 6815 lb. of dried WETF feed were successfully vitrified, producing respectively 5008 and 4362 lb. of stable glass product. Actual WETF glass produced met all disposal requirements with 95% confidence and achieved a projected full scale volume reduction (VR) ranging from 2.8:1 to 13.8:1, depending on the percent solids in the sludge. Operation of the vitrification unit proved highly reliable, achieving a 95% availability for the two week demonstration. The melter operated at 40% of the design capacity, which was based on the melting rate for SLS glass. Several process improvements have been identified to increase the throughput to near SLS capacity. Design improvements will also be made to reduce feed carryover into the off-gas system and the corrosion rate of the refractory.
WETF SLUDGE DESCRIPTION
Approximately 212,000 ft3 of WETF sludge is stored in four tanks at the WETF Tank Farm at the Y-12 Plant. The sludge is generated from biodenitrification treatment of uranyl nitrate solutions resulting from the uranium recovery process at Y-12 and lime-based precipitation of plating wastes. The sludge also contains wastes resulting from the clean-up of evaporative ponds and limited volumes from the hydroxide precipitation of metal bearing process wastes. Approximately 90% of the WETF sludge solids consist of calcium carbonate and biomass resulting from the biodenitrification process, with the remaining 10% consisting of hydroxide sludges.
The primary constituents of concern in the WETF sludge are hazardous heavy metals (cadmium, chromium, nickel, lead, and silver), volatile organic compounds (VOCs) (tetrachloroethylene, methanol, carbon disulfide), semi-VOCs (phenols, cresols), and radionuclides. The WETF sludge contains oil and grease in concentrations ranging from 0.35 to 2.0%, and moisture contents ranging from 60 to 90%. The WETF sludge is classified with RCRA waste codes F001, F002, F006, and F007 due to the influent sources to the WETF, and is a mixed waste due to the presence of uranium and thorium isotopes and Tc-99.
SLUDGE EXTRACTION
Approximately 2000 lb. of sludge was extracted from each of the four WETF tanks and placed in 55-gal drums. A vibrating core sampler was used (Fig.1) to extract sludge from three of the tanks containing sludge with 30% solids. The operation was conducted by lowering the vibrating sampler into a WETF tank through manways on top of the tank. After reaching the bottom of the tank, the vibratory mechanism was de-energized and a vertical core of the sludge was retained inside the sampler by a fingered catching device. When the sampler was raised from the tank, the catcher was removed, allowing gravity to expel up to 3.75 ft3 of sludge per penetration into a drum on top of the tank. The consistency of the sludge ranged from muddy water to peanut butter, with the viscosity increasing with sludge depth. The sludge was black in color with no apparent stratification layers.

Fig. 1. Vibrating corer extraction
setup.
In the fourth tank with low solids sludge(<10%), the sludge viscosity was too low to utilize the catcher in the vibrating sampler. Consequently, the sludge in this tank was extracted with a pneumatically operated diaphragm pump. The intake hose to the pump was attached to the bottom of the core sampler so that the intake could be vertically traversed across the sludge to obtain a representative sample.
BENCH SCALE TESTS
Objectives and Experimental
The bench scale test objectives were to:
To meet these objectives, a composite sample from four drums (one representing each WETF tank) was taken for use in the bench scale tests. This sludge was dried in a drum oven at 650°F to remove organics and free water prior to the vitrification crucible melts. The melts were performed in 150-g batches with a one to two hour residence time in a small muffle furnace.
The following variables were evaluated for their effect on glass processability and TCLP metals performance:
Additionally, refractory corrosion and devitrification were evaluated as a function of waste loading. At the conclusion of the bench tests, the goal was to have formulated a processable glass, which is defined as a glass composition that tolerates small changes in chemistry, has a low liquidus point devitrification product, melts at the desired temperature, and is not extremely corrosive to the refractory.
Bench Scale Results
The initial variable tested was the glass type, where the processability of borosilicate and SLS systems were compared. Initial borosilicate glass formulations either phase-separated or did not completely melt. SLS formulations produced homogeneous glasses within the target waste loading range, so the SLS system was selected as the basis for the target glass. Borosilicate glass formulations were not pursued further because of the narrow boundaries of phase separation in the glass composition, and the higher raw material costs versus SLS compositions. Borosilicates are typically used in high level waste applications because the boron acts as a neutron shield to attenuate dose while providing additional glass durability. Given the observed phase separation in the borosilicate glasses and the low dose rate of the WETF sludge, the traditional benefits of the borosilicate glass were not realized.
With an SLS system selected for the target glass, the next variable tested was lithium (Li) substitution for sodium (Na) as a fluxing agent. Baseline melts using only soda ash (Na2CO3) as the flux produced viscous glasses that were not completely melted at 2400°F, which indicated a need for a lower melting temperature composition that Li addition can provide. Based on compositions tested previously at Clemson University (1), a matrix of 11 SLS glass compositions was established to vary the molar ratios of Li to Na (1:0.55 to 1:4) and to determine the minimum amount of Li required to produce a homogeneous, completely melted glass. Minimizing the Li addition was driven by economic factors since Li is an expensive flux additive. Melts with a molar ratio of 1:2 Li:Na were acceptable for use in the demonstration test, forming a homogeneous, low viscosity glass at temperatures of less than 2450°F. With the Li:Na ratio set, the waste loading was varied in the range of 20 to 50%, which affected both the quality of glass produced and the resulting VR. The test indicated that acceptable glasses were formed at waste loadings ranging from 30 to 45%. Glasses above and below this range exhibited phase separation in the form of unmelted batch or a solidified molten salt.
Refractory durability and devitrification, two important parameters to system operability and life-cycle costs, were then evaluated using formulations at 25, 35 and 45% waste loadings. Inprevious work by SRS and ORNL (2), glasses incorporating high calcium-containing wastes such as WETF were determined to be very corrosive to refractory, possibly due to the very low viscosity of high calcium glasses. Mullite and flux refractory coupons were placed in glass samples for three hours at 2400°F; visible corrosion was only noted for the 45% waste loaded glass. Devitrification tendencies were determined by maintaining 25 to 40% waste-loaded glass samples at 1650°F for 14.5 hrs.. All samples devitrified at these conditions. The 40% waste-loaded devitrified samples melted and fused to the bottom of the crucible, indicating a low softening point for the glass or devitrification products, which would prove beneficial when operating or restarting a melter. The devitrified products were remelted at 2400°F and produced low viscosity glasses that readily poured, indicating that any devitrification occurring in the full scale melter could be remelted within the operating temperatures of the unit.
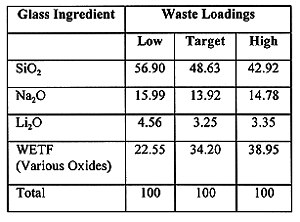
Based on the tests described above, a processable SLS glass formulation in the 30 to 40% waste loading range was targeted. Below 30% the glass was prone to phase separation and above 40% visible corrosion occurred in the refractory coupons. TCLP performance was confirmed by testing samples at the extremes of the operating window. Glass samples with 22.5% and 39% waste loadings were subjected to the TCLP test, and consistently met all metals leaching requirements. One sample at 22.5% waste loading did exceed the TCLP leachate concentration for lead, though this data point was considered an anomaly with lead leachate concentration over four times that of the next highest concentration. The demonstration test target loading was selected for 34%, which was closer to the high loading end to minimize life-cycle costs but conservatively distanced from the 45% loading, where phase separation and refractory corrosion were observed. The high, low, and target waste loading formulations are shown in Table I.
Table I WETF Bench Scale Glass Compositions (wt%)
PROOF-OF-PROCESS DEMONSTRATION TEST DESCRIPTION
Overview and Objectives
The proof of process demonstration test objectives were to:
A two ton/day, joule-heated melter was utilized to meet the above objectives. Prior to introducing the actual WETF sludge to the melter, three melter volumes of surrogate were processed to achieve steady state vitrification conditions, to confirm the processability of the selected glass composition and to minimize the amount of mixed waste generated during the demonstration test. The test was conducted from December 18 through December 30, 1995, during which a total of 13,826 lb of feed was successfully converted into 9,370 lb of stable glass product.
Melter Description
The Envitco EV-101 melter is a refractory-lined, high-temperature, cold-top vitrification system. The glass is heated by molybdenum electrodes which can operate at temperatures up to 2,700°F. Batch materials are fed in the top of the melter, cover the molten glass surface, and provide an insulating blanket. This "cold-top" operation is optimal for processing radioactive or mixed wastes, as the batch blanket layer condenses volatile species (e.g., Cs, Cd) and returns them to the molten glass. The melter is designed with a primary glass drain bay for flow control and temperature conditioning, a bottom metals drain for removal of elemental or secondary phase metals, and a salt drain bay for removal of low viscosity salts that can accumulate at the glass/batch interface. Gases and entrained particulates generated from the melting process are treated in a commercial off-gas system equipped with a quencher, venturi scrubber, wet electrostatic precipitator (WESP), and a HEAP filtration system.
Proof of Process Experimental
The 16 drums of WETF sludge were composited into four drying boxes with each box containing one drum from each of the four WETF tanks. The composited material was then dried at 650°F in a drum dryer. WETF surrogate and dried actual WETF sludge were combined with the glass forming ingredients and mixed in 500 lb batches in a portable electric concrete mixer. Three gallons of water mist spray were added to each batch as it mixed to reduce dusting and agglomerate fines. Batch was manually fed to the melter feed hopper in 5-gal buckets; the waste glass produced was collected in 55-gal drums in 79-gal overpacks with sand placed in the annular space between the two drums as insulation. The melter was operated 24 hr/day for the first part of the test and for 12 hrs/day in the latter portion of the test with a "hot hold" in the evening shift.
PROOF OF PROCESS RESULTS
Drying
The drum dryer reduced the moisture content to only 20% when drying the larger quantity of sludge. The outer layers of the WETF material formed an insulating layer, which prevented the sludge in the center of the box from reaching the temperatures necessary to drive off the water. The target formulation identified in the bench scale was maintained with no correction made for the residual water. Thus, the waste loading was reduced to 30%, slightly less than the target value (due to the higher moisture content), but the target waste loading was still within the acceptable TCLP performance range established in the bench scale.
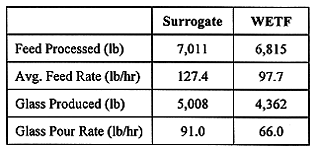
System Operability/Performance
Table II shows the demonstration test operations summary for batch processed and glass produced for both the surrogate and WETF. Melt rates for the surrogate WETF batch were typically 80 to 100 lb/hr, and operation required minimal operator adjustment. Maintaining stable batch cover and steady operating conditions proved difficult with the actual WETF batch,and more operator attention was required to counteract glass foaming and insufficient feed distribution on the melt surface. Stable operation was achieved by operating at higher feed rates and higher temperatures, resulting in melt rates of 60 to 80 lb./hr at 2500 to 2700°F; improvements to optimize the process and increase throughput are discussed later.
Table II Surrogate and WETF Processing Summary
Overall system operation proved to be robust, with an availability of 95% for 283 hours on-line. Downtime was experienced from a jammed rotary vane feeder and from localized devitrification in the glass drain. Devitrification occurred several times during "hot-hold" periods and in all cases, the devitrified product was quickly remelted during restart by applying additional energy to the drain area.
Glass Formulations
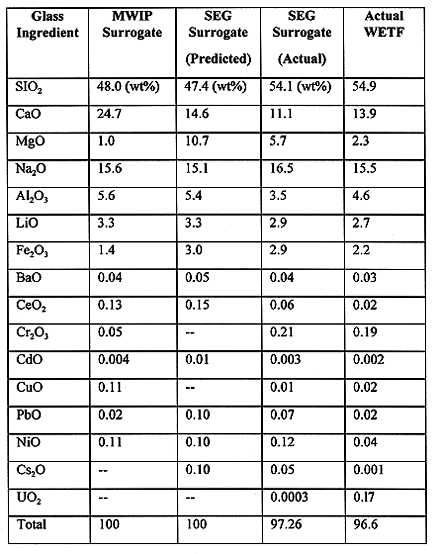
The WETF surrogate was based on the recipe developed as part of the DOE Mixed Wasted Integrated Program (MWIP) (3). Differences in the formulation include that dolomitic lime, a calcium magnesium carbonate, was used in the SEG recipe, rather than calcium carbonate. However, both calcium and magnesium behave similarly in the glass to provide durability and lower the melt viscosity. The target cumulative contribution of magnesium and calcium is approximately equivalent in both the MWIP and SEG formulations. Also, in the SEG surrogate, the concentration of lead and cadmium was increased to demonstrate the robustness of vitrification in processing higher heavy metal concentrations in the WETF sludge. Finally, nonradioactive cesium was added to the SEG surrogate to test the performance of the system in retaining volatile species in the melt.
Surrogate glass samples were taken throughout the process with the latter samples being most representative of steady-state operation. The last three sample analyses were averaged, normalized to account for recoveries (which were between 66 to 108% with an average of 90%), and are compared to the predicted SEG glass composition in Table III. The predicted "MWIP" composition presented is based on a glass produced with the MWIP surrogate recipe at the target 34% waste loading. The analysis of the glass produced from the actual WETF waste is also presented in Table III. The difference in predicted versus actual compositions is partially due to the lower than predicted waste loading, caused by the residual water in the dried waste. Sodium and lithium concentrations correlate well, while calcium, magnesium and other minor constituents contributed by the waste are typically low. High silica and chromium may be associated with refractory dissolution. No feed analysis was conducted, so mass balance calculations are not presented here.
Table III MWIP Surrogate, SEG Predicted Surrogate, SEG Actual
Surrogate, and Actual WETF Glass Formulas
Glass Waste Form Performance and Volume Reduction
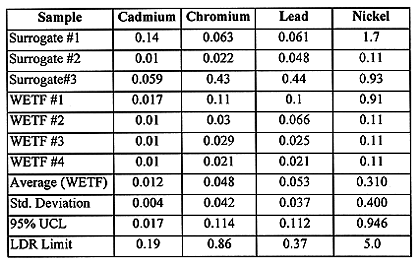
The glass waste form produced in the demonstration test met LDR with 95% statistical confidence. TCLP results show that the 95% upper confidence limit (UCL) for the WETF glass samples was three times lower than RCRA LDR. Table IV summarizes the 95% confidence statistical calculations for the primary RCRA metal constituents of concern. One of the surrogate samples failed the LDR limit for lead (0.37 mg/l) at 0.44 mg/l, but the surrogate, since it is not a listed waste, must only meet characteristic limits (5.0 mg/l for lead). The glass waste form also met the disposal site WAC for radioactivity, with the activity from each radioisotope being at least an order of magnitude lower than the disposal site limit.
Table IV WETF Primary Glass Waste Form TCLP Metals Result
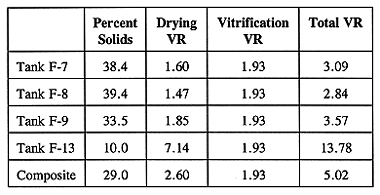
Volume reduction for sludges is highly dependent on moisture content. Since the historical moisture content from each tank was known, the VR from each tank was estimated based on the assumptions that the drying VR is proportional to the water removed, and that the density of the solids from each tank is the same. Under these assumptions, the drying VR will vary while the vitrification VR remains constant for the sludge in each tank. As shown in Table V, the total VR ranged from 2.8 to 13.8, with Tank F-13 yielding in the highest VR as it contains less than 10% solids. The vitrification VR for all tank sludge dried solids is estimated at 1.93.
Table V WETF Sludge Volume Reduction
Process Challenges
Activity within the melter (glass flow patterns, liquid formation, wetting of grains, melting of grains) during actual WETF processing was noticeably less than that of the surrogate. These differences can be attributed to several factors. First, since the WETF waste contained 20% residual moisture, energy was applied toward evaporating the water rather than melting batch. It is also likely that the actual WETF batch materials segregated because the drying technique employed for the WETF produced larger particle sizes than that of the glass forming ingredients. Separation of the smaller flux materials and the coarser, refractory WETF would occur during material handling (for example, loading the hopper and the batch drop from the charger). The segregated fines and fluxes then preferentially melt and join the bulk glass without adequately wetting the WETF grains. Additionally, the angle of repose for the WETF batch on the glass surface was much higher (likely due to the residual moisture and the particle size differences) than that of the surrogate, which lessened the surface area of batch exposed to the melt.
Dust generation and carryover to the off-gas system was higher than desirable and precluded the evaluation of the melter performance for retaining cesium (in the WETF surrogate) in cold top operation . Dust was generated from an approximate two foot drop from the rotary vane feeder to the melt. Fines generated were quickly entrained into the off-gas draft through the main off-gas port, located adjacent to the feed port.. Carryover was significantly reduced during the test by reducing the negative pressure on the melter from -0.3" to -0.05" water column, indicating that improved sealing of the superstructure would also reduce the draft and subsequent carryover.
Process Improvements
The focus during the test was to achieve steady, reliable operation of the system and demonstrate the performance of the glass waste form. Throughput of the process was not optimized due to the short duration of the test. Several process improvements have been identified to improve process stability and throughput to accommodate processing the entire inventory of WETF sludge. The use of a rotary vacuum dryer prior to vitrification will provide several benefits to increase the melt rate. First, the moisture content in the sludge will be reduced to less than 5%, which will minimize the evaporative load on the melter. The mixing action of the rotary vacuum dryer will overcome the insulating effects of static drying and provide the intimate contact and heat transfer necessary to dry the WETF completely. Also, the shear of the rotary blades will promote batch uniformity by reducing the WETF particles to a size distribution that can be matched more closely to the distribution of glass forming ingredients. The vacuum dryer design also provides the opportunity to blend the glass formers with the as-received WETF sludge. This maximizes the homogeneity of the batch by drying the waste in intimate contact with the glass formers and fluxing agents. Agglomeration of this dried batch will minimize segregation of the fluxes from the more refractory materials due to entrainment or material handling, and ensure uniform composition throughout the batch blanket.
Further improvements in throughput can be attained by increasing the glass flows beneath the batch blanket. This approach is used in commercial glass melting to increase melt rate and improve glass quality. A short test was conducted by bubbling air through a 3/8 in. black iron pipe inserted through the top of the melt. This bubbling improved distribution of the batch blanket and produced additional underlying glass flows. Both of these factors contribute to improved batch melt rates, process stability, and throughput.
Improved batch delivery to the melter offers an opportunity to reduce segregation and dust generation by minimizing the batch free-fall distance. Multiple point screw chargers allow for lower release points and can traverse the melt surface to improve the distribution and coverage of the batch blanket. Improvements in the batch blanket reduce volatile losses and heat losses, while improving melt rate and throughput.
Improved refractory performance will be required for production facilities to help maximize uptime and reduce life cycle costs. The 42% Al2O3, 54% SiO2, 0.6% alkali, slip-cast flux refractory used in this demonstration is not recommended for any extended period of melting with waste glasses. The flux refractory was provided with the original installation, because it is suitable for startup, testing and training operations where multiple cycles and thermal shock conditions normally occur. It is only moderately resistant to aggressive, low viscosity glass compositions, so high wear rates during this test were anticipated. Higher performance chrome or zirconia based materials are recommended for future, long term testing and operation. Such highly chemical resistant, chrome-bearing refractory was in place during the demonstration in the expected severe wear area between the main chamber and the glass drain. This refractory showed minimal wear upon disassembly of the unit.
CONCLUSION
Screening of glass formulations in the bench scale crucible melts yielded a processable glass and durable waste form. The bench scale formula selected was qualitatively scaleable to full scale melter operations for glass properties such as viscosity, operating temperatures, devitrification and remelting behavior. In the proof-of-process test, vitrification was demonstrated to be capable of treating the entire quantity of the WETF sludge, producing a stable glass product the met LDR and the disposal site WAC, and providing VRs ranging from 2.8 to 13.8. The throughput of the melter can be increased to about 150 lb per hour (near the two ton/day capacity for SLS glass) to accommodate full scale Phase II treatment through the following improvements identified to meet the challenges encountered in the demonstration:
In addition to implementing the above process improvements, further testing of the glass composition and processability of the glass needs to be conducted prior to operating at a commercial level. Tests include quantitatively determining:
REFERENCES