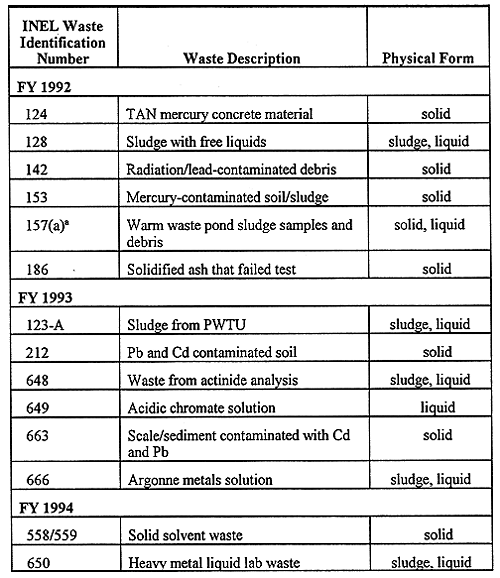
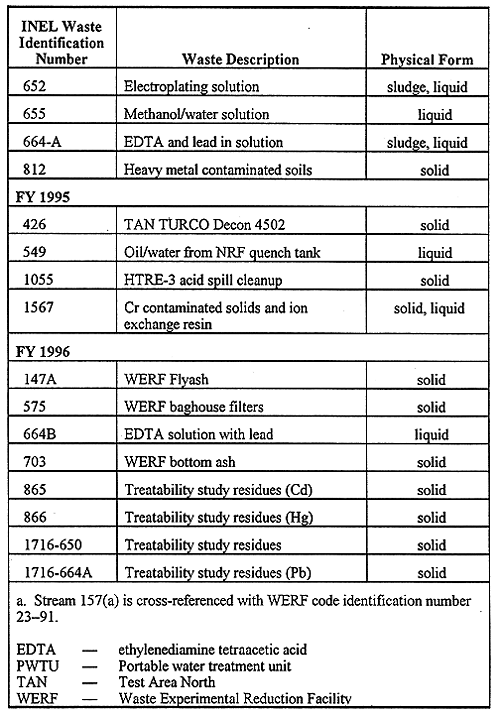
Table I. LDR Nonincinerable Mixed Waste Slated for Solidification.
Kevin L. Gering and G. Lynn Schwendiman
Lockheed Martin
Idaho Technologies Company
Idaho National Engineering Laboratory
P. O.
Box 1625
Idaho Falls, Idaho 83415-3625
ABSTRACT
This paper is a summary of five years of work involving bench-scale solidification of nonincinerable, land disposal restricted low-level mixed waste at the Idaho National Engineering Laboratory. The solidification studies performed for this work were done under Resource Conservation and Recovery Act treatability studies. Waste forms included liquids, sludges, and solids, and treatment techniques included the use of conventional hydraulic systems (Portland cement with and without additives), proprietary commercial formulations, and sulphur polymer cement. Solidification was performed to immobilize hazardous heavy metal constituents of concern (most notably mercury, lead, chromium, and cadmium), as well as small amounts of volatile and semivolatile organic compounds. Pretreatment options for mixed wastes are also discussed, utilizing a decision tree based on the form of mixed waste and the type of hazardous constituents.
Over the past five years, hundreds of small concrete monoliths were formed for a variety of waste types. The experimental parameters used for the hydraulic concrete systems include the ratio of waste to dry binder (Portland cement, proprietary materials, etc.), the total percentage of water in concrete, and the amount of concrete additives. The only parameter that was used for the sulfur polymer-based monoliths is ratio of waste to binder. Optimum concrete formulations or "recipes" for a given type of waste were derived through this study, as based on results from the Toxicity Characteristic Leaching Procedure analyses and a free liquids test. Overall results indicate that high waste loadings in the concrete can be achieved while the monolithic mass maintains excellent resistance to leaching of heavy metals. In our study the waste loadings in the concrete generally fell within the range of 0.5 to 2.0 kg mixed waste per kg dry binder. Likewise, the most favorable amount of water in concrete, which is highly dependent upon the concrete constituents, was determined to be generally within the range of 300 to 330 g/kg (30-33% by weight). The results of this bench-scale study will find applicability at facilities where mixed or hazardous waste solidification is a planned or ongoing activity.
INTRODUCTION
The objective of this paper is to discuss the bench-scale pretreatment and solidification of samples of low-level mixed waste (LLMW) at the Idaho National Engineering Laboratory (INEL), which has been done during five years of Resource Conservation and Recovery Act (RCRA) treatability studies. Solidification was performed on over thirty INEL wastes, where the treatment techniques included the use of conventional hydraulic-type cements (e.g., Portland cement), proprietary formulations, and a thermoplastic-type cement, sulphur polymer cement (SPC). Waste forms included liquids, sludges, and solids.
The ultimate goal of this work is to determine the optimal solidification techniques to transform low-level mixed waste to low-level nonhazardous waste by satisfying pertinent processing criteria and disposal requirements for the treated waste. In so doing, we are developing waste-specific treatment schemes for classes or groupings of LLMW. This work is meaningful in that it will provide a basis for the disposal of waste that is currently categorized as land disposal restricted (LDR) low-level mixed waste.
WASTE DESCRIPTIONS
Mixed wastes are those that contain both chemical and radiological hazards. The INEL mixed wastes that were investigated during this study are listed in Table I, wherein the waste code, waste name and physical form are given. Most of the wastes listed in Table I are being temporarily stored in approved containers (e.g. DOT 17-C drums or equivalent). As this table indicates, there has been a good representation of solid, liquid, and sludge waste forms that have been investigated during the span of our treatability studies. The treatability work for the FY 1992, 1993 and 1994 LLMW samples has also been discussed elsewhere (1).
Table I. LDR Nonincinerable Mixed Waste Slated for Solidification.


TCLP testing is a RCRA regulatory tool that is the criterion by which a waste sample is judged as hazardous or nonhazardous from a toxicity basis (2,3) and will be the primary focus for the analysis of heavy metals in treated samples for this work (see following section). For the majority of this study, the hazardous waste constituents of concern are heavy metals. TCLP results for toxic metals in the untreated samples are given in later tables.
Toxic organic constituents were present in a handful of the mixed wastes studied herein. Analyses were performed on the LLMW samples to quantify these volatile and semivolatile organic constituents. Overall, the hazard associated with organic constituents in our LLMW samples was small or nonexistent, depending on the waste code. Thus, the effect of solidification on organic materials in wastes will not be discussed herein.
Gamma ray, gross spectrometric alpha, and beta-emitter analyses of the mixed waste samples were performed by the INEL. Quantitative results are not given herein because the ultimate focus of this solidification study was to investigate the immobilization of toxic metals, not radionuclides, and since the treated waste under consideration will be disposed of as low-level radioactive waste. Generally speaking, the radioactivity of the LLMW samples was in the low pCi/g to low nCi/g range. Although there were some samples that contained transuranic components, their activity was sufficiently low to enable the waste samples to be classified as low-level mixed waste, not transuranic mixed waste.
REGULATORY GUIDELINES FOR LLMW TREATMENT
The development of mixed waste treatments performed at the INEL has been done under treatability studies approved by the State of Idaho. The EPA guidelines in the Code of Federal Regulations has designated the "treatability study" as means for developing treatments for hazardous waste. A treatability study is defined in 40 CFR 260.10 (4), as
"a study in which a hazardous waste is subjected to a treatment process to determine:
- whether the waste is amenable to the treatment process,
- what pretreatment (if any) is required,
- the optimal process conditions needed to achieve the desired treatment,
- the efficiency of a treatment process for a specific waste or wastes, or
- The characteristics and volumes of residuals from a particular treatment process.
Also included in this definition....are liner compatibility, corrosion, and other material compatibility studies and toxicological and health effects studies."
Treatability Studies are also Covered in 40 CFR 261.4.e-f (5)
Hazardous waste at the INEL is typically Characteristic or F-listed. Characteristic wastes are those which exhibit the characteristic of ignitability, corrosivity, reactivity or toxicity (6). F-listed waste typically involves spent solvents and other organic compounds. Most of the hazardous waste investigated herein carried the waste codes associated with corrosivity and toxicity for metals, such as cadmium, chromium, lead and mercury. The EPA has established treatment standards for toxic metals that must be met prior to land disposal of any treated waste (7). A summary of the treatment standards for the toxic metals is provided in Table II.
Table II. EPA RCRA Treatment Standards for Toxic Metals in TCLP
Leachate (3, 7).

SOLIDIFICATION BACKGROUND
General Information
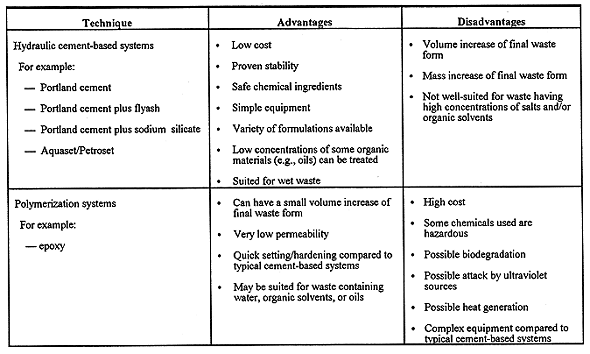
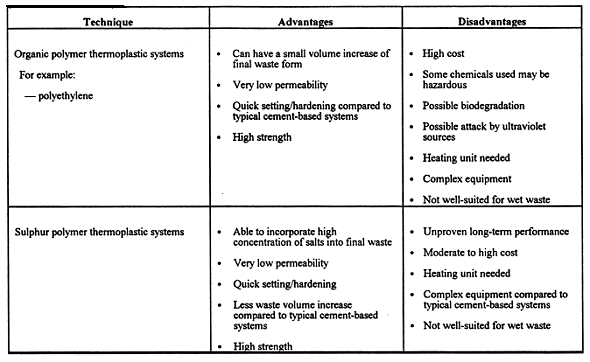
The bench-scale studies described herein employed a comparative technology screening as a basis for choosing solidification methods for the aforementioned INEL waste samples. There are several excellent references that gave guidance for this screening and other issues related to solidification/stabilization of hazardous or mixed wastes (8-16). Summary information from this screening process is given in Table III. The solidification techniques provided in Table III were evaluated by considering the compatibility between a proposed treatment and the LLMW physical and chemical characteristics. Also considered in our technology screening is the treatment cost per unit of LLMW, and the change of waste volume due to treatment (impacts disposal costs). It should be noted that the actual, specific solidification treatments that are used may depend on the type of required pretreatment (e.g., segregation, size reduction, pH neutralization, chemical precipitation, drying, etc.), and the final choice of full-scale solidification treatments may certainly depend on the cost of pretreatment options.
Table III. Candidate Techniques for Treatment by Solidification.
Bench-scale solidification was accomplished under controlled, supervised, and monitored conditions. Solidification took place in situ, where the cement and waste were mixed and cast in the same container, forming a homogeneous concrete mixture; this practice helped to reduce waste generation during bench-scale studies. The monoliths were formed inside containers (casts) having internal volumes that ranged from 400 mL to 4 liters. Most monoliths had volumes of slightly less than 1 liter. For hydraulic-type concretes, monolith containers were made of polymer material (e.g. high density polyethylene), whereas metal casts were used for SPC concretes. The use of metal containers is recommended for thermoplastic systems (e.g. SPC) because of the higher temperatures that are encountered. Care must be taken to heat the SPC in a well-ventilated area because of the potential liberation of small quantities of hydrogen sulfide (H2S) gas at temperatures exceeding 150°C (17).
Hydraulic Systems
The Portland cement used for this work was a Type I and II, low alkali formulation produced by either Ash Grove Cement West Incorporated or Holnam. This type of cement was used as a hydraulic binder in concrete to solidify liquid, solid, and sludge waste samples. Note that concrete is defined here as the mixture of binder, water, waste, and any additives. A number of concrete additives were used for various desired effects such as cure acceleration, enhanced metals immobilization, scavenging of excess water, etc.. Additives included sodium silicate pentahydrate, hydrated lime, ferrous sulfate, ferrous and ferric chloride, sodium phosphate dibasic, sodium sulfide, and proprietary materials (Aquaset I,II® and Petroset I,II®). De-ionized water was used as the make-up water in the concrete recipes. Hydraulic concrete monoliths were allowed to cure at least 28 days before they were sampled.
Non-hydraulic System (Sulfur Polymer Cement)
Sulfur polymer cement (SPC) is a non-hydraulic thermoplastic material that is gaining acceptance as a concrete-like binder material for waste treatment of dry hazardous wastes (13, 14, 16). One of the attractions of using molten SPC for waste treatment is that the free sulfur can react with available toxic metals within the waste particles, forming highly insoluble metal sulfides that are resistant to leaching. The SPC used for this work sells under the trade name CHEMENT 2000, and is in the form of flakes having a thickness of approximately 3 mm. This thermoplastic material is composed of approximately 950 g/kg sulfur and 5g/kg additives (dicyclopentadiene and oligomers of cyclopentadiene). SPC was used to solidify dry waste only, where mixed waste samples typically had to undergo a drying pretreatment. Monoliths made with SPC required only an overnight cure time, although there was typically at least a five-day period between SPC monolith production and sampling.
TREATABILITY STUDY EXPERIMENTAL PARAMETERS
The purpose of our matrix-based studies was to determine the most effective monolith "recipe" for each mixed waste. The parameters (variables) that define the experimental matrix in for this work include waste loading (weight ratio of LLMW to dry binder material), total water content of concrete mixture, type of cement binder, and the type of additives used (if any). The only parameter for the non-hydraulic cement portion of the study was the waste loading.
The most important variable in our monolith formulations was the waste loading, expressed as a weight ratio of LLMW to dry binder (kg LLMW per kg dry binder). In most cases the binder was Portland cement. Ideally, it is desirable to optimize the waste loading in concrete by determining the maximum amount of waste that can be incorporated into the concrete while satisfying disposal criteria (i.e. pass TCLP, no free liquids, etc.). In so doing, the final volume of the treated waste is minimized; hence, disposal costs are reduced. However, in practice it is recommended that the waste loading be slightly below the determined maximum to insure that a safety margin is in place. Values for waste loading were somewhat conservative in the first year of our treatability work, ranging from 0.2 to 0.6, then increased in the second through fifth years, having maximum values that often exceeded 1.0.
From a processing viewpoint, finding an appropriate water content for waste treatment is a balance between having concrete that is too thick during mixing (insufficient water) and concrete that produces a standing liquid layer upon curing (excess water). The total amount of water that is required depends on the net hydrophobicity or hydrophilicity of the concrete components (LLMW, hydraulic binder, and additives). Through the guidance of solidification references and trial and error, it was determined that the optimal total water content within the concrete generally fell within the range of 300 to 330 g/kg (30% to 33% by weight) for Portland cement-based recipes. Some of the early monoliths that contained 360 g/kg water (36% by weight) produced a thin liquid layer above the cured monolith; however, none of these early monoliths contained free liquids as determined by EPA Method 9095 (reference 18).
Under some instances it may be desirable to enhance the physical or chemical characteristics of concrete through the use of additives. As one example, this work investigated the use of sodium silicate pentahydrate (Na2SiO3•5H2O) as a means of providing greater immobilization of toxic metals. A concentration of 80 g/kg added Na2SiO3 was investigated during the first two years of our studies, whereas 20 and 40 g/kg were used during years three through five. Other additives used in our studies include hydrated lime, ferrous sulfate, ferrous and ferric chloride, sodium phosphate dibasic, sodium sulfide, Aquaset I,II® and Petroset I,II®.
PRETREATMENT CONSIDERATIONS
It was found in our treatability studies that LLMW pretreatment can have a profound effect on the ultimate success of the treatment method. For some waste forms the choice of pretreatment(s) is as important as the choice of the final treatment method. Waste pretreatment may include one or more of the following: segregation, size reduction, drying, mixing, neutralization, chemical precipitation, flocculation, clarification, etc. The decision tree given in Fig. 1 was developed in these studies for the characterization and pretreatment of LLMW. Pretreatment may be necessary to make a waste more compatible with a given solidification technique by increasing waste homogeneity and through canceling the inhibitive effects of particular waste constituents (e.g., acids, salts, or organic solvents).
For our bench-scale studies, pretreatment depended on the waste type and planned solidification treatment. For solid LLMW, pretreatment was primarily comprised of segregation/screening, size reduction of larger particles, mixing, and drying (for use with SPC). For liquid and sludge LLMW, pretreatment usually entailed pH adjustment, chemical oxidation/reduction, chemical precipitation, clarification, and dewatering (decantation). Pretreatment was not treated as a systematic test parameter; however, it was viewed as an important consideration in the overall solidification process.
EVOLUTIONARY CHANGES OF TREATABILITY STUDIES
Through the course of performing five years of solidification treatability studies, there was an inevitable evolution of our methodologies and treatment philosophies. In general, our approach to LLMW treatment was able to build on the knowledge and expertise gained from each successive year of treatability studies. The results are that waste-specific treatments have become more refined, more emphasis has been placed on pretreatment, and interim characterization has become more valued as a tool for decision-making. The primary benefit from this evolution is that we can estimate how much we can increase waste loadings without failing disposal criteria. As waste loadings are maximized the treated volumes are minimized, which has a direct impact on disposal costs.
Waste-Specific Treatments
A LLMW can be assigned to a treatment group according to the compatibility of the physical and chemical characteristics of the waste with the kind of treatment (see Table III). Stated simply, specific treatment methods are highly effective in treating well-defined types of wastes, and as such those treatments are waste-specific. Our treatability studies gave us a foundation of data that allowed us to match waste types with treatment methods. As more treatability work was done we were able to extend our capability to match waste types with specific concrete formulations, where we could exploit the knowledge gained from previous monoliths to target favorable monolith recipes for new wastes, while avoiding unpromising formulations.
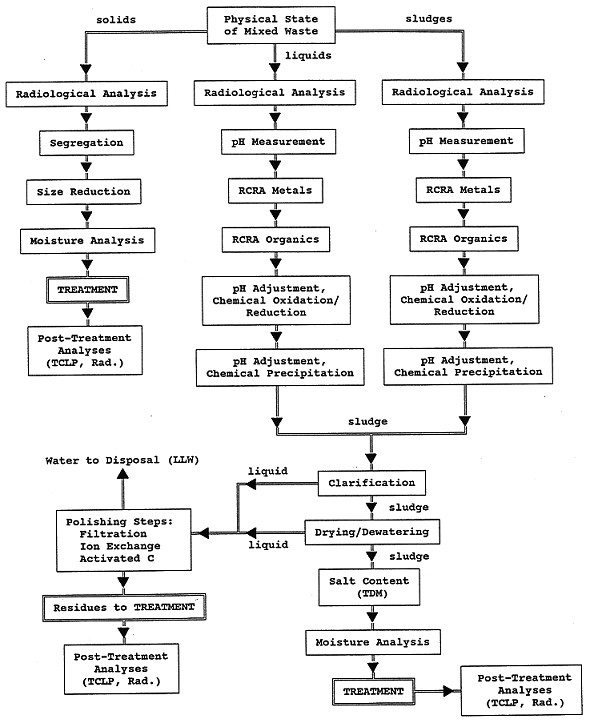
Fig. 1. Increasing emphasis on
pretreatment.
It is an understatement to say that pretreatment is important in solidification treatments, since the success or failure of a treatment method can hinge on the effectiveness of the chosen pretreatment(s). Early treatability work viewed pretreatment as somewhat optional, depending on the waste form. However, as more attention was given to the microscopic aspects of solidification processes, pretreatment was given a higher priority. In essence, a pretreatment step or series of steps acts to prepare the LLMW for favorable interactions with the binder material. The decision tree given in Figure 1 emerged out of our treatability studies as a pretreatment strategy to prescribe appropriate pretreatment steps for a given LLMW according to its physical and chemical characteristics.
Interim Characterization
Another aspect of our treatability work that evolved over the last five years is the use of interim characterization, that is, performing analyses on a LLMW while it undergoes a series of pretreatments and treatments. The net result of interim characterization is that a detailed picture of pretreatment/treatment effectiveness is gained. This detailed picture does much to uncover unsatisfactory conditions, particularly in the area of pretreatment, so that the approach can be revised until satisfactory results are obtained prior to final treatment. An example of interim characterization is to use atomic absorption analysis to monitor the level of dissolved toxic metals in a LLMW liquid that is undergoing a series of pH adjustments and chemical precipitation steps.
RESULTS
General Observations
Over 300 monoliths were made during the solidification treatability studies performed by the authors over the past five years. Approximately 82 percent of these monoliths passed the RCRA treatment standards (via TCLP testing) for toxic metals. No monoliths were observed to have free liquids as defined by EPA Method 9095 (18), although there was a small percentage of monoliths that had a thin layer of extruded liquid (standing water) that appeared during the curing phase. Most of the monoliths incorporated Portland cement as the binder and a smaller number of monoliths utilized proprietary formulations (e.g. Aquaset II®) or SPC as the binder.
TCLP Test Results
All waste-bearing monoliths were subjected to TCLP testing after they had undergone a curing period. TCLP data were generated by licensed offsite laboratories. Data validation and qualification are performed according to project needs. A summary of the TCLP results for the RCRA toxic metals is given in Table IV, where results are shown for the untreated (raw) LLMW, as well as the "best" results for the treated LLMW. Monolith formulations are indicated for the best cases. The results given in Table IV correspond to the concrete recipes that produce no free liquids, and that lower the leachability of toxic metals to the greatest extent while allowing the ratio of waste to binder to remain high. Although their choice may be somewhat subjective, the recipes indicated in Table IV are the best recipes deduced by this study as far as metal leachability and free liquids are concerned.
Table IV. TCLP Results for untreated and "best" treated
low-level LLMW samples. Values are in mg/L (in TCLP leachate) unless otherwise
noted. LLMW loading, water content, and additive data are given for the superior
concrete formulation for each waste code, as based on RCRA treatment standards,
free liquid test, and high waste loading within the concrete.
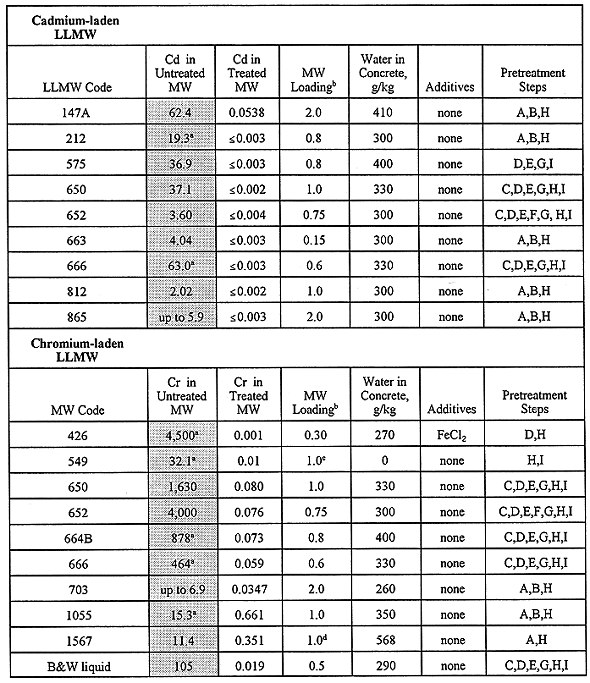
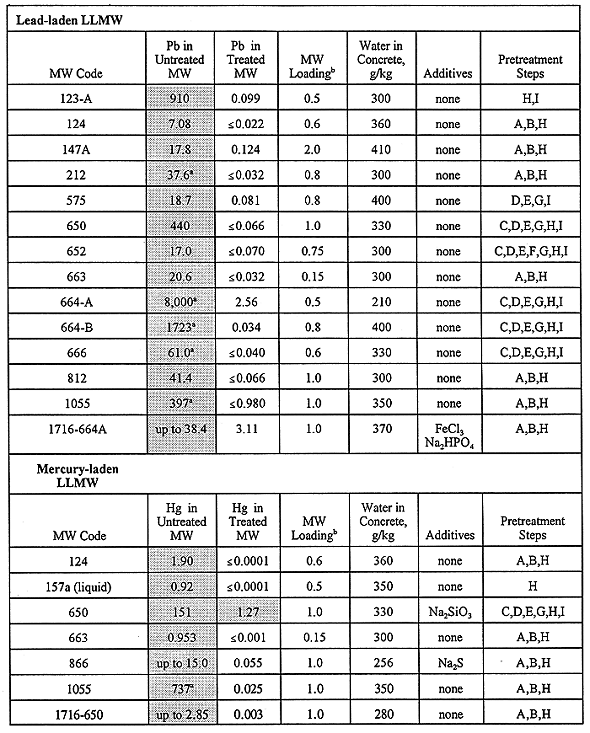
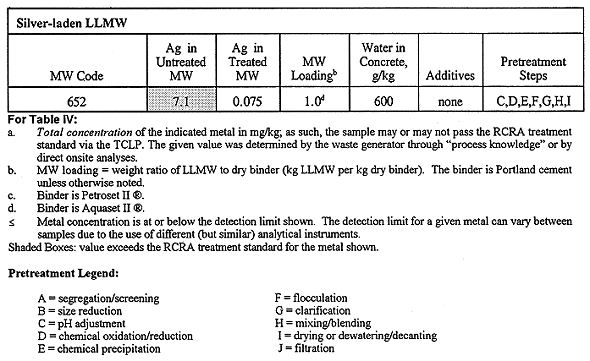
The results in Table IV indicate that solidification via Portland cement is a very effective means of immobilizing toxic metals, where monolith recipes generally have high waste loadings (most values 0.8) while decreasing the leachability of the hazardous metals to levels at or below detection limits. Such results infer that it may be possible to load the concrete with greater amounts of waste while passing TCLP tests and satisfying disposal criteria. It should be noted that Table IV gives clear indication of the toxic metals that are predominate at the INEL: lead, cadmium, chromium, and mercury. The lead and mercury in LLMW are largely artifacts of radiation shielding required during many years of operation at the INEL. Cadmium is found in INEL LLMW in part because it has been used at nuclear reactor facilities due to its unique transparency to some forms of radiation; routine cleaning and maintenance of such facilities will liberate small quantities of cadmium. Chromium-related LLMW is partly derived from various laboratory analyses and cleaning/decontamination activities that utilize chromic acid and sodium chromate.
Table IV also shows that the use of concrete additives were the exception to treatment schemes and not the norm. In general, a concrete additive(s) is not recommended for a final treatment formulation unless an obvious benefit has been exhibited by that additive. Thus, Table IV indicates that Portland cement alone provides satisfactory final treatment of most of the LLMW materials encountered in our five years of treatability studies, with appropriate pretreatments having been performed as noted in the table.
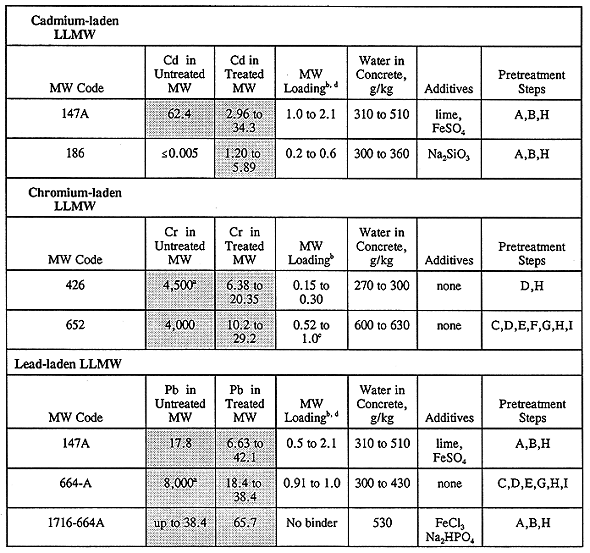
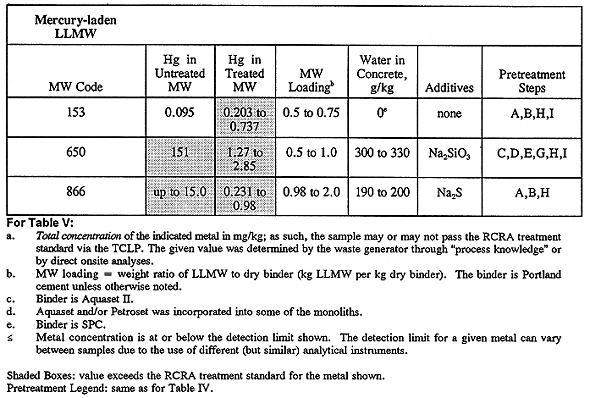
A small number of monoliths failed TCLP tests, wherein their metals concentrations exceeded the limits imposed by RCRA. Given the relatively broad experimental design matrix described herein, it should not be unexpected that some of the monoliths would fail one or more disposal criteria. The treated LLMW samples that failed TCLP are given in Table V, where it seen that monolith formulations failed the leaching tests for LLMW codes 147A, 153, 186, 426, 650, 652, 664-A, 866, and 1716-664A. Note that "LLMW" and "MW" are used interchangeably in Tables IV and V.
There are a few possible explanations why some of the monolith samples for INEL waste codes 153 and 186 failed TCLP tests while the untreated wastes passed. First, the laboratory results or procedures for the analysis of the metals of concern could be in error. Second, the concrete ingredients may have caused a chemically favorable environment for the leaching of the indicated metals, i.e. the ingredients may have promoted leaching of the metals. Lastly, the pretreatment step(s) used for INEL waste codes 153 and 186 may have altered the waste matrix and caused it to be more susceptible to the effects of leaching. Pretreatment of INEL waste code 153 involved drying the moist waste over a hot plate at 150180°C overnight, followed by size reduction to less than Þ in. via mortar and pestle. INEL waste code 186 was pretreated by size reduction only. All of the monolith recipes that failed TCLP for INEL waste code 186 contained added sodium silicate. The cause for these anomalous results should be found before full-scale solidification of these wastes is attempted.
Monoliths for LLMW codes 650, 664-A, and 1716-664A that failed TCLP tests probably failed due to the presence of ethylenediamine tetraacetic acid (EDTA) in these wastes. EDTA is a chelating agent that can prevent some soluble metals from participating in chemical precipitation and cementitious reactions. More discussion about the adverse affects of EDTA on solidification is given below.
Treated monoliths for 147A that failed TCLP for lead and cadmium may have been adversely affected by the hydrophobic nature of the raw waste, as it was observed that the LLMW particles for 147A were not easily wetted with water. It is suspected that there was a small fraction of heavy hydrocarbon residue in this flyash material (from incomplete combustion) that may have interfered with metals immobilization when hydraulic binders were used to treat this waste.
The monoliths that pass the disposal criteria tests (nonhazardous under RCRA; no free liquids, etc.) will be reclassified from LDR mixed waste to low-level nonhazardous waste. Treated waste that meets disposal criteria will be disposed of via a low-level waste disposal facility such as the Radioactive Waste Management Complex (RWMC) at the INEL. Those monoliths that fail to meet the disposal criteria will be kept in an approved temporary low-level mixed waste storage area, and re-solidified or encapsulated at a later date.
Free Liquids Test Results
When appropriate, a free liquids test was performed on samples of the cured monoliths in accordance with EPA Method 9095, Paint Filter Liquids Test (18), and the results indicate that none of the concrete formulations tested from our studies produced monoliths that had free liquids as defined by Method 9095. However, there were a small number of monoliths that had a thin layer of extruded liquid on top of the solidified concrete, which is common for hydraulic-type systems. This liquid layer appeared mostly in monoliths having a higher water content (>=360 g water per kg concrete), and rarely appeared at lower water contents (¾330 g/kg) . Realistically, this liquid layer could be decanted and set aside for further treatment if it contains toxic amounts of heavy metals, then used as process or makeup water for other solidification work. It is worth noting that none of the formulations containing added sodium silicate contained such a liquid layer, as the sodium silicate appears to have effectively bound the excess water within the concrete matrices.
Table V. Results for untreated and treated low-level LLMW samples
that failed TCLP for hazardous metals. Values are in mg/L (in TCLP leachate)
unless otherwise noted.
Pretreatment and Treatment Difficulties
In our studies, insufficient initial characterization was seen for many of the INEL LLMW codes mentioned herein. Up-front characterization is an important prerequisite for the successful pretreatment and final treatment of a mixed or hazardous waste. Up-front data should be up-to-date, detailed, and complete. The data that is required will vary according to the waste form and anticipated pretreatments and the final treatment.
EDTA is believed to have caused some monoliths of INEL 650, 664-A, and 1716-664A to fail TCLP tests. EDTA is an organic compound that acts as a chelating agent for metals in solution, causing them to stay soluble when they would otherwise form a solid precipitate. Thus, EDTA has an adverse effect upon chemical precipitation reactions as well as reactions involved with the setting of concrete. Seeing this, we sought ways in which we could pretreat our LLMW samples to render the EDTA ineffective as a chelating agent. For example, studies were performed on LLMW 664-B (very similar to 664-A) after the detrimental effects of EDTA were assessed in 664-A. We used KMnO4 -based chemical oxidation as well as ultraviolet-enhanced oxidation to oxidatively destroy the EDTA in 664-B, where the metals bound in the EDTA-metal complexes were released to form precipitates, thus reducing the concentration of hazardous metals in the liquid portion of the waste (19). The encouraging TCLP results in Table IV for waste code 664B (lead and chromium) show that the interfering effects of EDTA were reduced satisfactorily prior to final treatment.
Finally, some difficulty was had in using aluminum sulfate (Al2(SO4)3*18H2O) as a flocculating agent in INEL 652. This compound was used, following a chemical precipitation pretreatment step, as a means of accelerating the settling of solid precipitates. Upon the addition of approximately 10 g aluminum sulfate per kg LLMW 652, the levels of Ag, Cd, Cr, and Pb increased slightly in the liquid phase. As no appreciable benefit was seen from the use of this flocculation agent, its use was discontinued.
CONCLUSIONS AND RECOMMENDATIONS
This report has provided a summary description and evaluation of hydraulic cement-based and SPC-based solidification of mixed wastes generated at the INEL, as performed under bench-scale RCRA treatability studies. The basis of this evaluation is the ability of a given monolith recipe to satisfy pertinent disposal criteria, namely, TCLP and free liquids tests.
The results indicate that Portland cement systems can be used to successfully immobilize toxic metals in solid, liquid, and sludge mixed waste material. Of over 300 hydraulic cement-based monoliths produced, approximately 18 percent failed TCLP criteria for the toxic metals of concern. Only three SPC-based monoliths failed TCLP. Concerning free liquids testing, monoliths subjected to EPA Method 9095 (Paint Filter Liquids Test) showed no signs of free liquids. The data presented herein indicate that the most favorable concrete formulations are waste-specific, but overall have the following general composition: the waste loading (kg LLMW per kg dry binder) is between 0.5 and 2.0, with a total water content within the concrete of roughly 300330g/kg. This composition range takes into consideration the need to incorporate as much waste as possible into the monolithic form, thereby minimizing the solidified volume produced per unit of treated waste, while satisfying the waste disposal criteria for TCLP (hazardous RCRA metals) and free liquids. Such results infer that it may be possible to load the concrete with even greater amounts of waste while satisfying disposal criteria.
The addition of Na2SiO3 appears to be optional for most of the concrete formulations, considering the disposal criteria of passing TCLP and free liquids tests. This additive should be used only with good cause, as its use will result in greater treatment costs and greater disposal costs due to the small increase of the monolithic mass and volume that it causes. Finally, sodium silicate should not be added to concrete mixtures that contain cadmium-laden wastes (e.g. INEL waste code 186), as it appears to promote the leaching of cadmium from the treated waste form. Other additives (e.g. hydrated lime, some iron salts, sodium sulfide) provided benefits to concrete formulations, enhancing chemical and/or physical characteristics. However, these materials should only be used for full-scale LLMW treatment if the perceived benefits outweigh the added costs associated with their usage.
Wastes containing EDTA should be pretreated to eliminate the chelating action of this organic compound prior to any other pretreatment steps. Otherwise, heavy metals in solution may be bound to the EDTA and not able to participate in chemical precipitation and cementitious (concrete-forming) reactions.
In performing five years of LLMW treatability studies using solidification, an evolution of treatment methodologies and philosophies transpired. This evolution entailed an increasing emphasis on waste pretreatment, a greater reliance on interim characterization, and more refined waste-specific treatments.
Finally, this work is significant in that it demonstrates which concrete recipes succeed in passing disposal criteria, and which ones fail. The recipes that fail serve to define a set of limiting conditions (here, the concrete formulation) that can be used as a baseline for full-scale treatment of parent wastes or future solidification of similar wastes. The data contained herein could assist other DOE facilities that need to determine favorable methodologies and recipes for the treatment of their LLMW material and nonradioactive hazardous waste.
ACKNOWLEDGMENTS
This work was funded by the U.S. Department of Energy, Assistant Secretary for Environmental Management, Under DOE Idaho Operations Office, Contract No. DEAC0794ID13223.
REFERENCES