S. Amrit, P.E.; L. Baldy; E. Newberry, P.E.; and B. Kapoor,
CHMM
Bechtel National, Inc.
Oak Ridge, TN
ABSTRACT
Approximately 1,485kg of mixed waste (uranium oxides mixed with oil) at a Department of Energy site in Colonie, New York, contained elevated levels of extractable organic halides (EOX). The initial EOX concentration of the waste feed was 16,900 ppm. This waste was regulated under the Resource Conservation and Recovery Act (RCRA), and the land disposal restrictions criterion for EOX is 1,000 ppm.
To reduce the EOX concentration to an acceptable level, a solvent extraction process was deemed feasible because organic halides are in general miscible with organic solvents, while uranium oxides are not. The performance of three readily available and inexpensive organic solvents (isopropanol, diesel oil, and kerosene) in extracting the EOX compounds was evaluated. Bench-scale studies were conducted to assess the ability of each solvent (in the following order of priority): 1) to extract the EOX compounds, 2) to obtain a distinct phase separation, and 3) to obtain a clear extract phase. Bench-scale study evaluation and analytical results led to the selection of kerosene as the organic solvent.
The full-scale extraction system was a single-stage multiple contact scheme. The process generated two phases: the extract and the raffinate (product). The extract, consisting primarily of kerosene and oil, was the EOX-rich phase; it was shipped for offsite incineration. The raffinate consisted primarily of the uranium oxides and the residual oil. The efficiency of the solvent extraction process in removing EOX from the matrix was approximately 80-90 percent (by weight).
Using the mass balance principle, the weight of EOX in the extract and the raffinate was calculated. For a feed-batch size range of 135.5kg to 315.5kg, the theoretical EOX concentration in the raffinate relative to its weight was plotted, and nomographs were generated. The theoretical EOX concentration was multiplied by a solvent-specific, empirically derived correlation factor, , to estimate the actual EOX concentration in the raffinate.
These nomographs were used as guidelines to determine the performance of the solvent extraction process and eliminate the need for intermediate sampling of the product stream. Use ofthese guidelines reduced analytical costs by approximately $15,000.
BACKGROUND
A mixed-waste stream at a Department of Energy site in Colonie, New York, contained uranium oxides and oil in the ratio of 5:3 (by weight). Because uranium oxides are potentially pyrophoric, the waste was kept under oil by the former facility owner to prevent its exposure to air. The oil fraction was the source of extractable organic halides (EOX). This waste was regulated under the Resource Conservation and Recovery Act (RCRA), and the land disposal restrictions criterion for EOX is 1,000 ppm (1).
The amount of feed was about 1,485kg, and the EOX concentration was approximately 16,900 ppm (2). A solvent extraction process was used to extract the oil fraction from the feed and consequently reduce the EOX concentration.
BENCH-SCALE STUDY
To select the most appropriate solvent for extracting the oil from the feed, three solvents (isopropanol, diesel oil, and kerosene) were evaluated through a bench-scale study. Because the oil was the source of the EOX, extraction of the oil would extract the EOX. The following three performance indicators (in the order of priority), were used for selecting the optimal solvent:
The following process parameters were used in the study:
Explanation of the Three Performance Indicators
![]() =
[(mass of EOX in the feed - mass of EOX remaining in the raffinate) ÷ (mass
of EOX in the feed)]*100. Efficiency is reported as a percentage (%).
=
[(mass of EOX in the feed - mass of EOX remaining in the raffinate) ÷ (mass
of EOX in the feed)]*100. Efficiency is reported as a percentage (%).
Other Quantitative Observations
![]() =
_[(F + S) - (E + P)]/(F + S)_ * 100,
=
_[(F + S) - (E + P)]/(F + S)_ * 100,
where
F = mass of feed
S = mass of solvent
E = mass of extract
P = mass of raffinate (product)
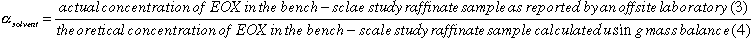
The solvent performance indicators and the quantitative observations obtained from the bench-scale study are reported in Table I (4).
Table I Solvent-Specific Performance Indicators and Quantitative
Observations
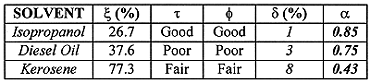
As this information shows,
![]() is
inversely proportional to
is
inversely proportional to
![]() .
The arithmetic product of the theoretical EOX concentration, EOXt,
and the correlation factor for kerosene,
.
The arithmetic product of the theoretical EOX concentration, EOXt,
and the correlation factor for kerosene,
![]() K
= 0.43, gives a fairly accurate estimate of EOX in the raffinate, EOXe
:
K
= 0.43, gives a fairly accurate estimate of EOX in the raffinate, EOXe
: ![]() K
* EOXt = EOXe
K
* EOXt = EOXe  actual
concentration of EOX in raffinate as reported by offsite laboratory, EOXa.
actual
concentration of EOX in raffinate as reported by offsite laboratory, EOXa.
Selection of the Solvent
Based on the evaluation of these performance indicators for the three solvents, kerosene was selected as the appropriate solvent for the process.
FULL-SCALE SOLVENT EXTRACTION PROCESS
"Like dissolves like." To extract organic halides (EOX) from the mixed-waste feed, an organic solvent, kerosene, was selected. The solvent extraction process generated two phases: the extract, and the raffinate (product). The extract, primarily solvent and oil, was the EOX-rich phase. The primary constituents of the raffinate were the uranium oxides that could not be extracted and the residual oil. The process goal was to obtain the lowest possible concentration of EOX in the product (raffinate) using a multiple-contact solvent extraction system operated in batch-mode.
Process Setup
The extraction system was a single-batch, multiple-contact scheme. Figure 1 shows the process schematic of the mixer-settler configuration for the system.
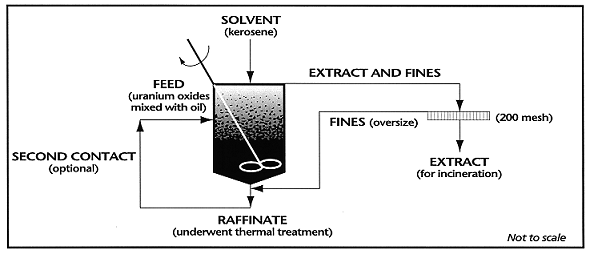
Fig. 1. Mixer-settler configuration.
Equipment
Process Parameters
Note: The full-scale process solvent feed ratio was increased beyond what was used in the bench-scale study in an effort to enhance the EOX removal efficiency.
Process Steps
The following steps describe the extraction process:
EOX Extraction Efficiency and System Performance
When kerosene was used as the solvent, the EOX removal efficiency was approximately 80 percent (by weight) with single contact and up to approximately 90 percent (by weight) with double contact. However, the EOX concentration in the raffinate (product) did not meet the regulatory criterion of <1,000 ppm.
The waste feed contained high concentrations of EOX. Solvent extraction was considered to be the optimal primary treatment technology option to extract the EOX from the waste feed because organic halides are, in general, extractable with organic solvents. The raffinate from this solvent extraction process was subsequently thermally treated (secondary treatment technology) to meet the treatment criteria.
MATERIAL BALANCE CALCULATIONS
Based on the feed weight, the initial EOX concentration (EOXi) in the feed, and the densities of the two feed components, uranium oxides and oil, the respective weight fractions of the two components were calculated to be 0.63 and 0.37. The following mass equivalence relationship was determined (Refs. 5 and 6):
59.2 kg of feed 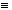 37.4 kg of uranium oxides
37.4 kg of uranium oxides
 21.8 kg of oil
21.8 kg of oil 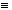 1.0 kg of EOXi.
1.0 kg of EOXi.
(The ideal result of this solvent extraction process would be that the mass of the raffinate approaches the mass of the uranium oxides.)
DEVELOPMENT OF MASS BALANCE NOMOGRAPHS
Based on the mass balance equivalence relationship, the theoretical EOX concentration in the raffinate, and the correlation factor for kerosene, the nomographs were plotted to depict the estimated EOX concentration in the raffinate versus the mass of the raffinate. Figure 2 is a plot of the nomographs for feed batch sizes from 135.5kg to 315.5kg.
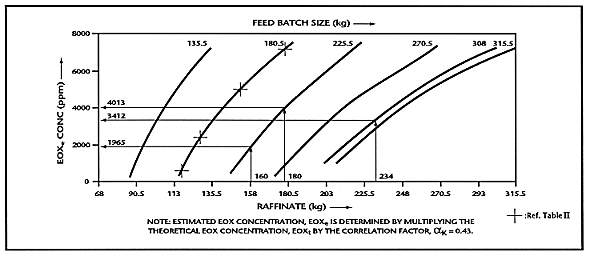
Fig. 2. Mass balance nomographs for
solvent extraction process for various feed batch sizes (solent: kerosene).
The nomographs were generated and used as guidelines to determine the performance of the solvent extraction process. A sample calculation explaining the generation of the nomograph for a feed batch size of 180.5kg is shown below in Table II:
| Feed batch size: | 180.5 kg | |
| Uranium oxides in the feed: | 0.63*180 kg | = 113.72 kg |
| Oil in the feed: | 0.37*180 kg | = 66.80 kg |
| EOX in the feed, EOXi | (1÷59.2)*180 kg | = 3.05 kg |
| Raffinate (product) = uranium oxides + residual oil | ||
| EOXt concentration = [EOX in the raffinate, kg ÷ mass of raffinate, kg]*106 = 16,900ppm | ||
| EOXe =
| ||
Table II Raffinate Analysis for Feed Batch Size of 180.5 kg
The numerical values of the raffinate, in kg, and the corresponding numerical values of the EOXe concentrations are indicated in the nomograph (Fig. 2) representing the feed batch size of 180.5kg.
VALIDITY OF THE USE OF THE CORRELATION FACTOR,
![]() K
K
Samples collected from the three batches of raffinate were sent to an offsite laboratory to determine actual EOX concentration (EOXa). The EOXe (obtained from the nomographs, Fig. 2) in each batch was compared with the corresponding EOXa; the summarized data are reported in Table III.
Table III Comparison of EOXe vs. EOXa for Various Feed and Raffinate Mass Values
EOXe was plotted against EOXa as shown in Fig. 3. As the plot demonstrates, there is a significant linear correlation (R2 = 0.80) between EOXe and EOXa, and the straight line passes through the origin. Also, it is shown that the theoretical estimate of the EOX is at least 0.85 times (very nearly equal to) the actual EOX concentration.
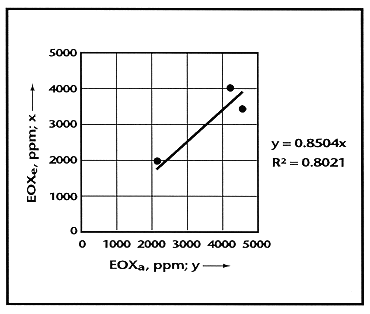
Fig. 3. Linear correlation between
EOXa and EOXe.
LIMITATIONS
The use of the mass-balance principle to accurately predict the contaminant
concentration is limited to phase separation processes like this solvent
extraction case study that yield distinct phases and have no intermediate
phases. The use of a correlation factor such as
![]() K
is solvent- and process-specific, and its accuracy depends on the measurement
accuracy of the masses of the four material components (F, S, E, and P) involved
in the process.
K
is solvent- and process-specific, and its accuracy depends on the measurement
accuracy of the masses of the four material components (F, S, E, and P) involved
in the process.
DISCUSSION
For every feed batch, a particular weight of raffinate corresponded to a hypothetical EOX concentration in the raffinate. A fairly accurate estimate of the EOX concentration in the raffinate was determined by multiplying the theoretical EOX concentration by the correlation factor 0.43 for kerosene.
The nomographs were designed to be used as guidelines to help determine the performance of the solvent extraction process. Use of these nomographs eliminated the need for intermediate sampling of the product to measure the process performance and resulted in savings of approximately $15,000 in analytical costs.
REFERENCES
ACKNOWLEDGMENT
*This work was successfully completed under Department of Energy Contract DE-AC05-91OR21949.