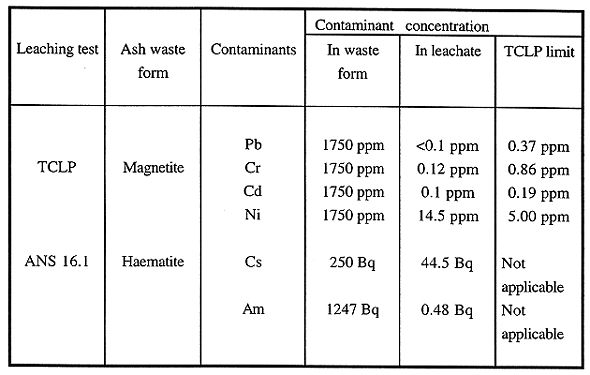
Table I. Leaching Studies on Ash Waste Form
A. S. Wagh, S. Y. Jeong, D. Singh
Argonne National
Laboratory
Argonne, IL 60439
A. S. Aloy, T. I. Kolytcheva, E.N. Kovarskaya
V. G.
Khlopin Radium Institute
St. Petersburg, Russia
Yevgeny (Jenya) Macheret
U. S. Department of Energy
Idaho Operations Office
Idaho Falls, ID 83401-1563
ABSTRACT
In an effort to develop chemically bonded phosphate ceramics for mixed waste stabilization, a collaborative project to develop iron-phosphate based ceramics has been initiated between Argonne National Laboratory and the V. G. Khlopin Radium Institute in St. Petersburg, Russia. The starter powders are oxides of iron that are generated as inexpensive byproduct materials in the iron and steel industry. They contain iron oxides as a mixture of magnetite (Fe3O4) and haematite (Fe2O3). In this initial phase of this project, both of these compounds were investigated independently. Each was reacted with phosphoric acid solution to form iron phosphate ceramics. In the case of magnetite, the reaction was rapid. Adding ash as the waste component containing hazardous contaminants resulted in a dense and hard ceramic rich in glassy phase. On the other hand, the reaction of phosphoric acid solution with a mixture of haematite and ash waste contaminated with cesium and americium was too slow. Samples had to be molded under pressure. They were cured for 2-3 weeks and then hardened by heating at 350°C for 3 h. The resulting ceramics in both cases were subjected to physical tests for measurement of density, open porosity, compression strength, phase analyses using X-ray diffraction and differential thermal analysis, and leaching tests using toxicity characteristic leaching procedure (TCLP) and ANS 16.1 with 7 days of leaching. Using the preliminary information obtained from these tests, we evaluated these materials for stabilization of Department of Energy's mixed waste streams.
INTRODUCTION
The chemically bonded phosphate ceramic (CBPC) process has been developed at Argonne National Laboratory (ANL) for nonthermal treatment of low-level mixed wastes (1). Mg-phosphate-based ceramic waste forms have shown excellent Land Disposal Restrictions (LDR) performance. However, MgO used in this process must be precalcined adding to the cost of powder preparation. Earlier work by Kingery (2) and more detailed Russian work (3,4) indicated that it may be possible to use iron oxides to form these ceramics by reacting the oxide with phosphoric acid solution. Because of the abundance of iron oxide as a natural mineral and as part of steel industry waste streams, the cost of waste stabilization will be much reduced if these oxides could replace MgO. In addition, iron phosphate based CBPCs will provide a way to produce value-added structural products with recycled steel and ferrous mineral industry wastes. For this reason, development of iron phosphate based CBPCs are being pursued in collaboration between ANL and V. G. Khlopin Radium Institute (KRI) in Russia, with funding and guidance from the U.S. Department of Energy. This presentation provides the preliminary development of these phosphate ceramics and their performance for stabilization of simulated mixed waste. It is hoped that this will open up the exciting possibility of using iron and steel industry wastes, such as slags that contain haematite (Fe2O3) and magnetite (Fe3O4), as well as waste phosphoric acid from fertilizer and petrochemical industries, to stabilize DOE waste streams and also to develop useful value-added low-cost structural products.
DEVELOPMENT OF IRON PHOSPHATE CBPCS
In his early work, Kingery (2) showed that haematite when reacted with H3PO4 takes 72 h to set into a tacky product, but magnetite reacts rapidly with a considerable exothermic reaction to form ceramic. Also Golynko-Volfson et al. (3, 4), reported that it is possible to calcine heamatite at 600°C and react with H3PO4 to form a ceramic. To explore these avenues, two independent but preliminary studies were initiated. At ANL, the route of magnetite was pursued while KRI pursued the haematite route. Ash was used in both the studies as the waste. The ash used at ANL was a simulated mixture of fly ash, bottom ash, activated carbon, and vermiculite. This mixture was spiked with nitrates of hazardous metals such as Pb Cd, Cr, and Ni so that the content of each metal in the final waste was 0.5 wt.%. Such an ash composition produces a surrogate of a typical DOE ash waste stream. The details of preparation of this surrogate waste may be found in a DOE report (5). The ash used in the Russian work was a real low level radioactive waste stream.
The ultimate objective of these two studies was to merge the findings and develop very versatile ceramics in which setting rates could be controlled at will and also, since haematite is more abundant and cheaper than magnetite, reduce the cost of forming these ceramics through the proper choice of the starting powders. Here we report these preliminary studies on the two independent routes of formation of the iron phosphate based ceramic waste forms.
a) Formation of CBPCs with magnetite:
Analytical-grade magnetite, obtained from Fisher Scientific was reacted with 50 wt.% dilute H3PO4 solution in a beaker. The mole ratio of the P2O5 to the powder was 1:1. The powder was stirred in the solution for 5 min and then immediately transferred to polyethylene syringes. With an exothermic reaction, the reacted slurry set to form dense ceramic with very glossy surface, indicating that it was rich in glassy phase. The samples were cured for 3 weeks before they were tested.
The ash waste forms were made in a similar manner. Ash was added to the magnetite powder in a ratio of 7:3. The ash used in this study contained: fly ash (40%), coal botton ash (33%), vermiculite (20%), and activated carbon (5%). The mixture was then reacted with the H3PO4 solution as before and the ceramic waste forms were formed.
b) Formation of CBPCs with haematite
Haematite is less reactive then magnetite. Therefore, in the beginning we made an iron phosphate mixture based on haematite and 50% solution of H3PO4 (mole ratio P2O5:Fe2O3 = 1.67:1). This mixture was placed in a closed can for one week at 50°C; to this iron phosphate binder we then added the ash waste. The mass ratio of Fe2O3: Ash = 5:3. This ash contained hydroxyapatite, quartz, haematite, calcite, calcium, carbonate, vitlokite, and an amorphous phase. According to chemical analysis, the content of carbonate ions CO3-2 in the ash was 13%. To make the ceramic, we used the fraction of ash that was of size < 0.25 mm. Radioactive chemical composition of the ash in Bq/gm was Am241 = 594, Cs137 = 119.
The paste of iron phosphate and ash was stirred for 24 h in order to have a more complete dissociation of carbonates; it was then poured into molds. After 24 h, the hardened samples of diameter 25 mm and height 10 mm were easily removed from the molds. They were dried in air until constant weight was obtained after 1 week. Then they were heated at 350°C for 3 h. The resulting samples were subjected to various tests. We also made samples without ash to study the composition of the matrix phase.
PHASE COMPOSITION OF CERAMICS AND WASTE FORMS
Both magnetite- and haematite-based CBPCs were glass-crystalline ceramics, and hence it was difficult to determine the phases. In the magnetite based ceramic, other than those of the unreacted magnetite, no peaks were observed and only a broad hump due to an amorphous phase was noticeable. In the ash waste form, on the other hand, even the peaks of magnetite disappeared and only a very broad hump was seen. This indicates that even the excess magnetite reacted in this waste form. Similar investigations were conducted on haematite-based CBPCs. In this ceramic, distinct peaks were obtained for some phases in the X-ray diffraction pattern and were confirmed by differential thermal analyses.
The phases observed in the haematite-based ceramics were FeH3(PO4)2.H2O and FePO4.H2O, both of which are being phases of Fe3+. Earlier Russian work on mixtures of magnetite, haematite, and wüstite (4) show that in addition to these two phases, other phases are Fe3(PO4)2.8H2O, and Fe(H2PO4)2.H2O, both phases of Fe2+. Possible haematite-based ceramics form phases with Fe3+, while when magnetite and wüstite are present, the ceramic also contains phases of Fe2+. This implies that it is quite likely that the rapid setting of magnetite may be due to Fe2+, while Fe3+ in haematite produces ceramics that set very slowly. More investigations will be pursued in this project to exploit this finding by controlling the rate of reaction through a proper combination of the two.
PHYSICAL PROPERTIES OF CBPC AND ASH WASTE FORMS
Density of the waste forms was measured by determining the mass and volume of geometrically regular samples. Open porosity was determined by the water intrusion method. The density and open porosity of haematite-based waste forms was found to be 1.17 g/cm3 and 62 vol.% respectively, while these for magnetite-based waste forms were 1.6 g/cm3 and 11.7 vol.%. These densities and porosities did not differ in the magnetite-based matrix material (1.7g/cm3 with nearly 11% open porosity). All had densities between 1.5 and 1.6 g/cm3 for magnetite ash waste forms, and porosity was slightly lower than that of the matrix material (i.e.,between 10 and 11 vol.%). We can assume that the high open porosity of the haematite samples is related to acid dissolution of calcium carbonates in ash and entrapment of CO2 bubbles by wastes with high carbonates, which produce gas in the formation of phosphates; thus we suggest suitable pretreatment.
These results show that the magnetite ceramic waste forms were denser materials. Due to the lower porosity of these materials, their compression strength was high, 5534±536 psi, which is comparable to that of conventional portland cement. Due to high porosity, the heamatite-based waste forms were weak and hence their strengths could not be measured consistent from sample to sample.
LEACHING BEHAVIOR OF CONTAMINANTS IN WASTE FORMS
In the case of magnetite-based waste forms, we performed the Toxicity Characteristic Leaching Procedure (TCLP) (6) tests to study retention of hazardous contaminants. Similarly haematite-waste forms were subjected to ANS 16.1 (7) by submerging samples in deionized water for 7 days and testing the leachate water for concentrations of radioactive contaminants. The results are given in Table I. Columns 4-6 of the Table also contain concentrations of the contaminants in the waste form, together with the Environmental Protection Agency's TCLP limits for the hazardous contaminants to be met as a part of the Land Disposal Restriction (LDRs).
When the concentrations of hazardous contaminants in the magnetite-based waste forms are compared with their counterparts in the leachate, one may conclude that these waste forms immobilize the contaminants extremely well except for Ni. The reason for the poor result for Ni is its slow dissolution in the acid. Since the ceramic sets too quickly, Ni does not react and form an insoluble phosphate completely. This will be remedied by retarding the rate of setting as we have done it in. All the numbers in the leachate are below the TCLP limits given in column 6, indicating that magnetite-based ceramic waste forms pass the LDRs extremely well. For ceramics based on haematite during 7 days of leaching, the loss for Am241 was 0.04%, while Cs leaching was 17%. Loss of matrix mass was 1.8 %. It is possible that fixation of Cs137 in the matrix can be improved by varying the molar ratio of P2O5:Fe2O3. The haematite-based waste forms, however, immobilize the radioactive contaminants well. Because there are no prescribed limits on leaching of the radioactive contaminants, and a superior performance of a waste form is judged by requiring that no contaminant should be observed in the leachate, performance of haematite-based waste forms needs further improvement.
Table I. Leaching Studies on Ash Waste Form

MECHANISM OF IMMOBILIZATION
In our earlier work on magnesium-based CBPCs (1), we showed that superior immobilization of hazardous and radioactive contaminants is attained by chemical conversion of the contaminants into their insoluble phosphate forms, followed by their physical microencapsulation in the dense physical matrix of the Mg phosphate.
The study presented here reveals these mechanisms further. In both magnetite- and haematite-based waste forms, the contaminants are converted to their insoluble phosphate forms. This chemical immobilization is responsible partially for the low leaching levels shown in column 5. In the magnetite based waste form, however, in addition to the chemical immobilization, physical microencapsulation in the dense ceramic is also attained. The scanning electron photomicrograph shown in Fig. 1 reveals this dense structure of the ceramic. One may notice a very glassy matrix, which might be coating the chemically immobilized contaminants just as they have encapsulated the cenospheres of ash in the micrograph. Such microencapsulation leads to extremely superior immobilization of the contaminants in the magnetite-based waste forms. On the other hand, due to the porous matrix of the haematite-based waste form, microencapsulation of the contaminants is not effective and hence the leaching levels of Am and Cs are higher. Thus it is necessary to improve the haematite-based waste forms by possibly incorporating Fe2+ phase in it so that it will not only accelerate the rate of setting but also provide a denser waste form.
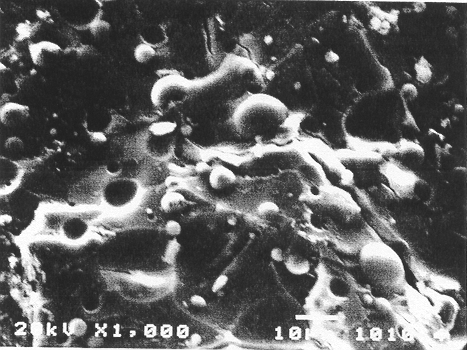
Fig. 1 Scanning electron
photomicrograph of ash waste form
CONCLUSIONS
This preliminary study demonstrates the potential of iron-based phosphates to stabilize mixed (hazardous and radioactive) waste streams. While magnetite-based waste forms are superior to haematite, the abundance of haematite as a raw material (as a component in soils as well as in waste streams), makes it an attractive candidate for further development as a waste form material. If good waste forms can be made with this material, several advantages may be achieved. These include the very low cost of waste stabilization and inexpensive value-added construction materials.
The results for magnetite-based waste forms indicate how this may be done. Fe3+ reacts slowly while Fe2+ reacts too rapidly. Thus, to produce viable methods of waste stabilization and practical ceramic waste forms, we must strike a balance between the two. This main finding of this study will be pursued to develop such ceramics in the future in this collaborative project between ANL, DOE, and KRI.
REFERENCES
*Work supported by the U.S. Department of Energy, Office of Technology Development, as part of the Mixed Waste Integrated Program, under Contract W-31-109-Eng-38.