STABILIZATION OF CONTAMINATED SOIL AND WASTEWATER WITH
CHEMICALLY BONDED PHOSPHATE CERAMICS*
A. S. Wagh, S. Y. Jeong, D. Singh, R. Strain, H. No, and J.
Wescott
Argonne National Laboratory
Argonne, IL 60439
ABSTRACT
At Argonne National Laboratory, we have developed chemically Bonded
phosphate ceramic (CBPC) technology to stabilize the U.S. Department of Energys
problem mixed waste streams, for which no other stabilization technology is
suitable. In this technology, solid waste is mixed with MgO and reacted with
aqueous solutions of phosphoric acid or acid phosphates at room temperature to
form a slurry that sets in 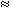 2 h into a hard and
dense ceramic waste form. Initial studies involved stabilizing the surrogate
waste streams and then testing the waste forms for leaching of contaminants.
After achieving satisfactory performance of the waste forms, we next
incorporated actual waste streams at bench scale and produced waste forms that
were then tested with the Toxicity Characteristic Leaching Procedure (TCLP).
This presentation deals with stabilization of soil contaminated with Cd, Cr, Pb,
Ag, Ba, and Hg, and of low-level radioactive wastewater. To enhance the
contaminant levels in the soil, we further spiked the soil with additional
amounts of Cd, Cr, Pb, and Hg. Both the soil and the wastewater were
incorporated in the same waste form by stabilizing them with the CBPC process.
The waste forms had a total waste loading of
2 h into a hard and
dense ceramic waste form. Initial studies involved stabilizing the surrogate
waste streams and then testing the waste forms for leaching of contaminants.
After achieving satisfactory performance of the waste forms, we next
incorporated actual waste streams at bench scale and produced waste forms that
were then tested with the Toxicity Characteristic Leaching Procedure (TCLP).
This presentation deals with stabilization of soil contaminated with Cd, Cr, Pb,
Ag, Ba, and Hg, and of low-level radioactive wastewater. To enhance the
contaminant levels in the soil, we further spiked the soil with additional
amounts of Cd, Cr, Pb, and Hg. Both the soil and the wastewater were
incorporated in the same waste form by stabilizing them with the CBPC process.
The waste forms had a total waste loading of 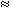 77
wt.% and were dense with an open porosity of 2.7 vol.% and a density of 2.17
g/cm3. Compression strength was 4910 psi. The TCLP results showed
excellent immobilization of all the RCRA metals, and radioactive contaminant
levels were below the detection limit of 0.2 pCi/mL. Long-term leaching studies
using the ANS 16.1 procedure showed that the retention of contaminants is
excellent and comparable to or better than most of other stabilization
processes. These results demonstrate that the CBPC process is a very superior
process for treatment of low level mixed wastes; we therefore conclude that the
CBPC process is well suited to the treatment of low-level mixed waste streams
with high waste loading.
77
wt.% and were dense with an open porosity of 2.7 vol.% and a density of 2.17
g/cm3. Compression strength was 4910 psi. The TCLP results showed
excellent immobilization of all the RCRA metals, and radioactive contaminant
levels were below the detection limit of 0.2 pCi/mL. Long-term leaching studies
using the ANS 16.1 procedure showed that the retention of contaminants is
excellent and comparable to or better than most of other stabilization
processes. These results demonstrate that the CBPC process is a very superior
process for treatment of low level mixed wastes; we therefore conclude that the
CBPC process is well suited to the treatment of low-level mixed waste streams
with high waste loading.
INTRODUCTION
The chemically bonded phosphate ceramic (CBPC) process was developed at
Argonne in response to a need at U.S. Department of Energy sites to treat "problem"
low-level mixed wastes by non-thermal processes (1). These waste streams are
secondary waste streams that are either off-gases from the thermal treatment of
waste streams or residues left after thermal destruction of organic streams.
Such waste streams cannot be treated by any thermal process again and hence need
a process that stabilizes their contaminants at room temperature.
In our earlier work, the CBPC process was shown to treat such waste streams
very effectively (1-3). Surrogate waste streams of ash containing hazardous
contaminants and cerium as a surrogate of U and Pu, were shown to form hard and
dense waste forms with excellent performance in short- and long-term leaching
tests.
The process consists of reacting a mixture of MgO powder and the solid waste
with a solution of H3PO4 or an acid phosphate. The
aqueous slurry, formed by mixing these components for 30 min, is a homogeneous
paste that hardens into a ceramic with mild exothermic reaction in approximately
2 h. The resulting ceramic waste form has a density of
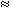 1.8 g/cm3 and an open porosity of
2-5 vol. Compression strengths of such ash ceramics are 2 to 3 times that of
portland cement. The Toxicity Characteristic Leaching Procedure (TCLP) (4) tests
have shown that the leaching levels are 1-2 orders of magnitude lower than the
U.S. EPA Land Disposal Restrictions (LDRs) limits. Accelerated leaching tests
such as the American Nuclear Societys ANS 16.1 (5) showed that retention of
contaminants is far superior to that of other similar methods such as cement
stabilization.
1.8 g/cm3 and an open porosity of
2-5 vol. Compression strengths of such ash ceramics are 2 to 3 times that of
portland cement. The Toxicity Characteristic Leaching Procedure (TCLP) (4) tests
have shown that the leaching levels are 1-2 orders of magnitude lower than the
U.S. EPA Land Disposal Restrictions (LDRs) limits. Accelerated leaching tests
such as the American Nuclear Societys ANS 16.1 (5) showed that retention of
contaminants is far superior to that of other similar methods such as cement
stabilization.
Encouraged by this superior performance of the CBPC waste forms, we tested
the process on actual waste streams at bench scale. In this attempt, we
demonstrated that one could stabilize a solid and a liquid waste stream
simultaneously. The solid waste stream was contaminated hazardous soil and the
liquid waste was U contaminated wastewater both from Argonne-E site. These two
were stabilized in a ceramic waste form of 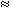 100
g. Its properties and leaching performance were tested to demonstrate that the
CBPC process is an excellent approach for treating actual wastes; details are
given below.
100
g. Its properties and leaching performance were tested to demonstrate that the
CBPC process is an excellent approach for treating actual wastes; details are
given below.
FABRICATION OF WASTE FORM
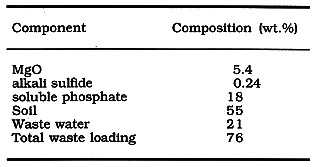
Contaminated soil, MgO, the phosphate powders, and a measured amount of
waste water were mixed together in a plastic container. With a spatula, the
mixture was stirred for 30 min. We also added a small amount of an alkali
sulfide powder for efficient stabilization of Hg. Initially, the slurry cooled
by few degrees when phosphate powder crystals dissolved in the water, but after
30 min, was back to room temperature and formed a paste with the consistency of
engine oil. The amounts of each component used in this mixture are given in
Table I.
Table I. Composition of the Slurry
The slurry was allowed to set for  2 h
during which it warmed up steadily. The maximum temperature recorded was
2 h
during which it warmed up steadily. The maximum temperature recorded was
 65°C. At 55°C, however, it set into a
hard mass. After several hours, it cooled as the heat dissipated.
65°C. At 55°C, however, it set into a
hard mass. After several hours, it cooled as the heat dissipated.
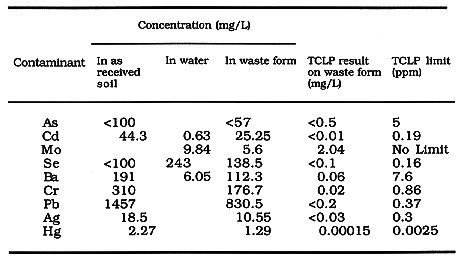
Table II Stabilization of Unspiked Soil and Water
We made two sets of samples. The first one was with the as-received waste
streams. Because this soil had very low concentration of some of the hazardous
metals, we spiked it with Cd, Cr, Hg, and Pb to test the process for higher
levels of the contaminants, and made a second set of samples. The soil was
spiked with soluble nitrates of Cd, Cr, and Pb, and chloride of Hg. The detailed
concentrations of the 'as-received' soil and the waste water are given in
columns 2 and 3 of Table II, and those for the spiked soil are given in Table
III. All of the hazardous contaminants that were part of either the soil or the
wastewater, exist in the waste form at significant levels. These levels are
given in the column 4 of Tables II and III.
Table III Data on Stabilization of Spiked Soil and Water
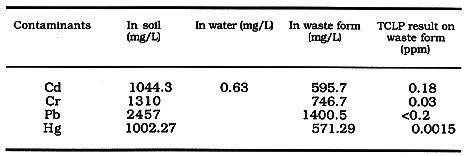
LEACHING PERFORMANCE TESTS
After curing the samples for 3 weeks they were subjected to Toxicity
Characteristic Leaching (TCLP) Studies (4). The results are given in columns 5
of Table II and III, while the EPA limits are given in the 6th column of Table
II for comparison.
A comparison of columns 5 and 6 show that the leaching levels are well below
the EPA standards in both Tables. Even the results on spiked samples given in
Table III show that stabilization has been very effective.
Table IV. Radioactive contaminants
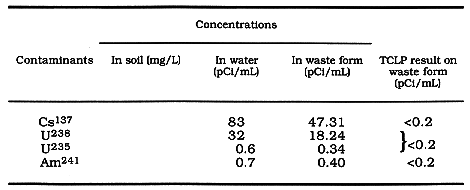
Table IV contains the leaching results on the radioactive contaminants. The
levels of Cs137, U, and Am241 are significant in the
water and hence in the waste form. However, no contaminants were detected in the
leached water at the detection limit of 0.2 pCi/mL.
Table V. Long-term leaching (ANS 16.1) results on waste form
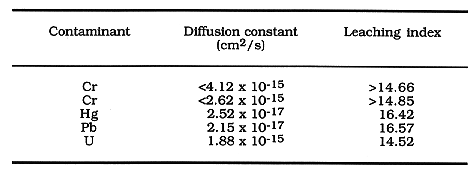
The American Nuclear Societys ANS 16.1 Standard Test (5) was followed to
evaluate the diffusion constants for the various contaminants in the spiked soil
and waste water form. Samples were immersed in leachants of deionized water;
results are presented in Table V. The determined leachability indexes range from
14.52 for U to as high as 16.57 for Pb, significantly better than the
recommended criterion of 6 (6). These results are further evidence of the
superior containment characteristic of the CBPC final waste forms.
Some insight has been gained on the stabilization mechanisms since our
earlier study. Chemical conversion of the contaminants such as Pb, Cd, and Cr
into their insoluble phosphate forms followed by their microencapsulation in the
dense ceramic matrix, leads to such superior stabilization in the phosphate
matrix. Hg, on the other hand, is stabilized into its sulfide form.
Investigation is underway on the other remaining metals to identify the exact
stabilization mechanisms.
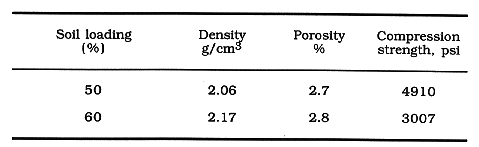
PHYSICAL PROPERTIES OF WASTE FORMS
Physical properties of the simulated soil waste forms were investigated. The
results are given in Table VI. Density of the waste forms is approximately 20%
lower than that of portland cement products. Open porosity is also extremely
low, while compression strength is comparable to that of portland cement
products. The low porosity clearly contributes to good encapsulation of the
contaminants in the waste forms, while the lower density and good compression
strength makes the waste form a lightweight but strong product for
transportation and storage.
Table VI. Physical properties of simulated soil waste form
CONCLUSIONS
This study on real waste has demonstrated that hazardous and radioactive
contaminants can be efficiently stabilized in the CBPC matrix by this
room-temperature process. In particular, the following findings are very
attractive for use of this method in stabilizing aqueous and soil waste streams.
- Contaminated soil and wastewater can be successfully stabilized in the same
waste form, thereby increasing the total waste loading to
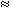 77 wt.%. To our knowledge, no other
low-temperature process can achieve such a high waste loading.
77 wt.%. To our knowledge, no other
low-temperature process can achieve such a high waste loading.
- Due to consolidation of the soil in the waste form, the waste form volume
was nearly the same as that of the soil. Thus, the net volume decrease was equal
to that of wastewater or 21 vol.%.
- TCLP results showed that leaching levels of all hazardous contaminants are
well below the most severe EPA testing standards. This ensures that the process
is very rugged and is tolerant to variations in contaminant levels in the waste
streams.
- TCLP results on radioactive contaminants are below the most severe
detection limits of the instrument. This ensures that no detectable leaching of
the radioactive contaminants occurs.
Due to the low temperature nature of the process, its simplicity, and its
volume reduction, we believe that use of this process will provide considerable
cost savings in waste management operations.
REFERENCES
- A. S. WAGH, D. SINGH and J. CUNNANE, Phosphate-Bonded Ceramics for
Stabilizing Problem Low-Level Mixed Waste, Annual Report for FY 94 on TTP
2-4-20-04 to Mixed Waste Integrated Program of DOE, Washington, DC (1994).
- A. S. WAGH and D. SINGH. Low Temperature-Setting Phosphate Ceramics for
Mixed Waste Stabilization. In Proc. 2nd Intl. Symp. & Exhibition on
Environmental Contamination in Central & Eastern Europe. Budapest, (1994)
pp. 633-635.
- D. SINGH, A. S. WAGH, S. Y. JEONG, and M. DORF, Leaching Behavior of
Phosphate Bonded Ceramic Waste Forms, To appear in Proc. 1996 Annual Meeting of
Amer Ceram. Soc., Indianapolis, IN, April 14-17, 1996.
- U.S. ENVIRONMENTAL PROTECTION AGENCY, Method 1311, Toxicity Characteristic
Leaching Procedure (TCLP), March 15, 1992, Rev. II, pp. 138-139.
- AMERICAN NUCLEAR SOCIETY, American National Standard Measurement of the
Leachability in Solidified Low-Level Radioactive Wastes by a Short-Term Test
Procedure. Method ANSI/ANS 16.1-1986, American Nuclear Society, La Grange Park,
IL, 1986.
- J. MAYBERRY, T. L. HARRY and L. M. DEWITT. Technical Area Status for
Low-Level Mixed Waste Final Waste Forms. Vol. I, DOE/MWIP-3 (1992).
*Work supported by the U.S. Department of Energy,
Office of Technology Development, as part of the Mixed Waste Focus Area, under
Contract W-31-109-Eng-38.
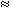 2 h into a hard and
dense ceramic waste form. Initial studies involved stabilizing the surrogate
waste streams and then testing the waste forms for leaching of contaminants.
After achieving satisfactory performance of the waste forms, we next
incorporated actual waste streams at bench scale and produced waste forms that
were then tested with the Toxicity Characteristic Leaching Procedure (TCLP).
This presentation deals with stabilization of soil contaminated with Cd, Cr, Pb,
Ag, Ba, and Hg, and of low-level radioactive wastewater. To enhance the
contaminant levels in the soil, we further spiked the soil with additional
amounts of Cd, Cr, Pb, and Hg. Both the soil and the wastewater were
incorporated in the same waste form by stabilizing them with the CBPC process.
The waste forms had a total waste loading of
2 h into a hard and
dense ceramic waste form. Initial studies involved stabilizing the surrogate
waste streams and then testing the waste forms for leaching of contaminants.
After achieving satisfactory performance of the waste forms, we next
incorporated actual waste streams at bench scale and produced waste forms that
were then tested with the Toxicity Characteristic Leaching Procedure (TCLP).
This presentation deals with stabilization of soil contaminated with Cd, Cr, Pb,
Ag, Ba, and Hg, and of low-level radioactive wastewater. To enhance the
contaminant levels in the soil, we further spiked the soil with additional
amounts of Cd, Cr, Pb, and Hg. Both the soil and the wastewater were
incorporated in the same waste form by stabilizing them with the CBPC process.
The waste forms had a total waste loading of 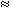 77
wt.% and were dense with an open porosity of 2.7 vol.% and a density of 2.17
g/cm3. Compression strength was 4910 psi. The TCLP results showed
excellent immobilization of all the RCRA metals, and radioactive contaminant
levels were below the detection limit of 0.2 pCi/mL. Long-term leaching studies
using the ANS 16.1 procedure showed that the retention of contaminants is
excellent and comparable to or better than most of other stabilization
processes. These results demonstrate that the CBPC process is a very superior
process for treatment of low level mixed wastes; we therefore conclude that the
CBPC process is well suited to the treatment of low-level mixed waste streams
with high waste loading.
77
wt.% and were dense with an open porosity of 2.7 vol.% and a density of 2.17
g/cm3. Compression strength was 4910 psi. The TCLP results showed
excellent immobilization of all the RCRA metals, and radioactive contaminant
levels were below the detection limit of 0.2 pCi/mL. Long-term leaching studies
using the ANS 16.1 procedure showed that the retention of contaminants is
excellent and comparable to or better than most of other stabilization
processes. These results demonstrate that the CBPC process is a very superior
process for treatment of low level mixed wastes; we therefore conclude that the
CBPC process is well suited to the treatment of low-level mixed waste streams
with high waste loading.





