A METHOD FOR REMOVING AND RECOVERING MERCURY FROM
OFF-GASES
Daryl L. Roberts and Tom E. Broderick
ADA Technologies
Dennis Sparger
Sandia National Laboratory
ABSTRACT
ADA Technologies is developing a sorbent-based process that recovers mercury
from flue gases, a process that regenerates the sorbent and recovers the mercury
in a form suitable for further distillation and ultimate recycle. Because of
these attributes of the process, ADA Technologies has adopted the name "Mercu-RE"
to describe its process.
In the laboratory, the uptake of mercury and mercuric chloride has been
tested with sorbents purchased commercially and prepared by ADA Technologies. In
addition, the sorbents have been imbibed into the body of ceramic filters to
simultaneously remove mercury vapors and particulate matter from flue gases. In
these tests, the mercury uptake efficiency has varied between 65% and 99%. The
efficiency of uptake was unaffected by the presence of eight volume percent
water vapor and HC1 concentrations of 50, 100, and 200 ppmv. In our early
laboratory work, selected sorbents and filters were regenerated thermally and
put through five cycles of sorption and regeneration. No loss of performance was
observed through these cycles.
Proof-of-concept field testing to date has included a two actual cubic foot
per minute (ACFM) unit at IT Corporation (Oak Ridge, TN) and a 50 ACFM unit at
Science Applications International Corporation in Idaho Falls, ID. In these
tests, the process removed over 99% of the mercury. The sorbent was not
regenerated in the two ACFM test but was regenerated three times during the 50
ACFM test (one week duration).
Current work is emphasizing the long-term durability of the sorbent.
Fundamental studies of the sorbent have shown that on some sorbents the noble
metal crystallites that comprise the active ingredient are thermally stable
after 2160 hours of exposure to the regeneration temperature of 700°F. This
time period represents about 540 days of commercial operation (four hours of
regeneration per day of operation). These thermal stability tests are
continuing, and long-term testing in the presence of aggressive flue gas
components is planned for FY '97.
INTRODUCTION
Mercury contamination is problematic in many DOE site remediation and waste
disposal efforts (Kvartek et al., 1994; Perona and Brown, 1993; Taylor, et al.,
1995). The most visible of these efforts is that of the Oak Ridge Y-12 plant
from which approximately 350 metric tons of mercury were discharged to the
environment between 1953 and 1963 (Turner, et al., 1984). These discharges have
led to a wide-ranging variety of soils and sediments that need remediation. In
addition, DOE has identified 38,000 m3 of mixed low-level and
transuranic wastes in its waste inventory that contain radioactive mercury
contamination, including some radioactive elemental mercury.
Retorting and vitrification are two leading options for treating
mercury-containing soils and wastes. These processes create a mercury-containing
off-gas that can contain a mercury concentration equal to the vapor pressure of
elemental mercury at the flue gas temperature (approximately 3500 ppmv or 20
grams per cubic meter at 300°F). Even the off-gas of a condenser
operating at the relatively low temperature of 50°F can have a mercury
partial pressure of 5x10-4 torr or 5,800 µg/m3.
Although there is no national standard for the concentration of mercury in all
exhaust gases, the Environmental Protection Agency regulates municipal waste
incinerators to a limit of 85 µg/m3. With respect to workplace
or public exposure, the Threshold Limiting Value for mercury is 50 µg/m3,
and the Immediately Dangerous to Life and Health (IDLH) limit is 28,000 µg/m3.
The IDLH value is the vapor pressure of mercury at approximately 80°F which
is why any fugitive liquid mercury in a lab, school, or office building is
regarded as an immediate threat. Finally, mercury exists in many chemical forms,
some of which are equally volatile to elemental mercury (e.g., the vapor
pressure of mercuric chloride at 300°F is 2 torr compared to 2.7 torr for
elemental mercury vapor).
Only municipal waste incinerators are governed by national regulations
today, and the best available control technology specified for municipal waste
incinerators is the injection of activated carbon. Improvements to this method
are needed since 100 to 10,000 pounds of activated carbon are required to remove
one pound of mercury from the flue gas depending on the concentration and
speciation of mercury in the flue gas. The mercury-contaminated carbon becomes
part of the ash collected by particle control devices. Recent studies at ADA
Technologies and other laboratories indicates that mercury re-volatilizes from
carbon, although the rate is slow at room temperature.
ADA Technologies has been developing a mercury control technology that aims
to remove mercury from flue gases and recover elemental liquid mercury as a
by-product with no solid wastes. The following paper reviews the status of the
control technology and gives an example of a field test wherein surrogate,
mercury-containing wastes were melted in a plasma hearth pilot unit under the
sponsorship of the Department of Energy, Office of Environmental Management.
BASIS OF ADA'S MERCURY CONTROL TECHNOLOGY
The underlying principle of ADA's regenerable mercury removal process
(Mercu-RE process) is the sorption of mercury and its common compounds by noble
metals and the regeneration of these metals by thermal desorption of the
mercury. This principle has been exploited for mercury analysis and
environmental sampling (Dumarey, et al., 1985; Slemr, et al., 1979). Simply
stated, mercury and its common compounds sorb to the noble metals and are
desorbed as elemental mercury vapor at an elevated temperature. In analytical
chemistry or environmental sampling work, this regenerability essentially
imparts an ability to concentrate the mercury from the low levels found in most
samples. It is then easily measured, either gravimetrically or by UV absorption.
In laboratory work to date, aimed at waste incinerator applications, ADA has
devised sorbents, essentially gold dispersed on either activated carbon or
alumina support particles, that remove up to 99% of elemental mercury vapor and
mercuric chloride vapor at 300°F in a synthetic flue gas containing more
than 1000 µg/m3 of mercury, 8% water vapor, and either 100 ppm
HC1 or 2000 ppm SO2. Further, upon heating to 500°F to 700°F,
the mercury is fully desorbed as mercury vapor, even when the mercury is sorbed
as mercuric chloride. We have also imbibed the sorbent into the walls of a
filter and performed mercury uptake and regeneration experiments. In sorbent
beads or in a filter form, the reversible capacity of the gold for mercury
exceeds 100 mg of Hg per gram of gold (10 wt.%).
In a typical experiment, approximately four grams of sorbent are held in an
oven, and the synthetic flue gas is passed through the bed with a superficial
velocity near 0.5 to 1.0 ft/sec. Typical sorbent pressure drops have been in the
range of 0.2" to 5" of water. Typical bed residence times have been in
the range 0.1 sec to 1.0 sec. The space velocities, a measure of throughput in
catalytic technologies, have been in the range of 10,000 hr-1 to
100,000 hr-1.
Representative results are depicted in Figs. 1 through 4. In Fig. 1, a
gold-spiked carbon bed sorbs essentially all of the mercury for more than 24
hours before mercury vapor breaks through. In Fig. 2, a gold-spiked alumina
sorbent takes up mercury for approximately 40 hours. The breakthrough shoulder
is broad; the capture efficiency falls below 90% after 10 hours but stays above
85% for the next 30 hours.
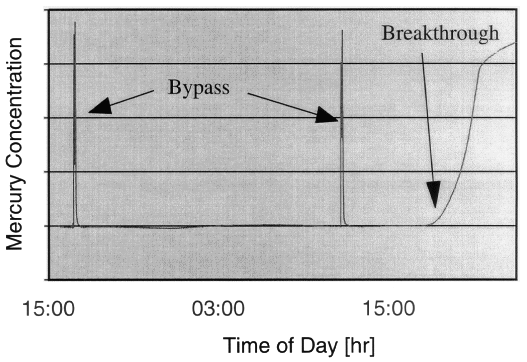
Fig. 1. Carbon-based sorbent removes
all mercury for 24 hours.
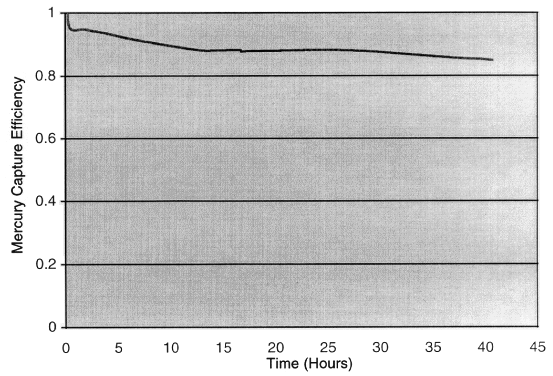
Fig. 2.Alumina-based sorbent removes
85% of mercury for 40 hours.
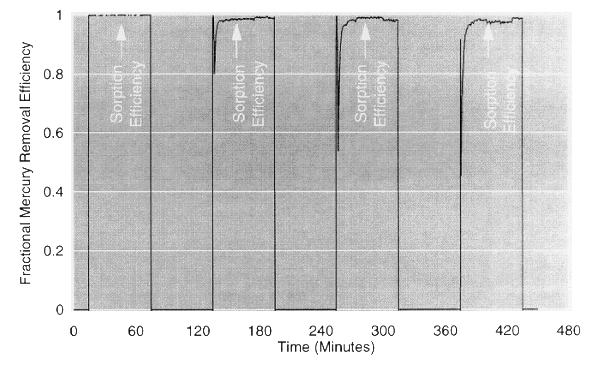
Fig. 3.Repeated regeneration of
alumina-based sorbent.
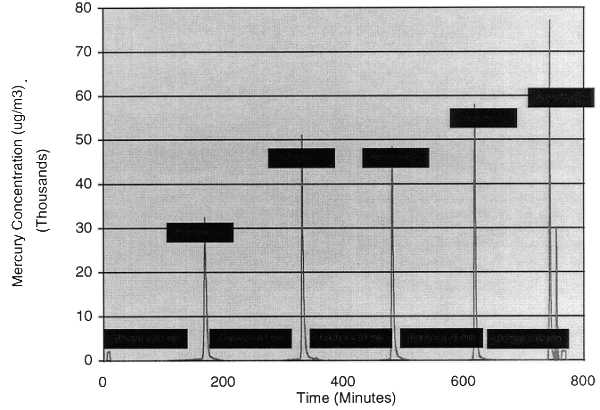
Fig. 4. Repeated regeneration of
sorbent imbibed into ceramic filter.
In Fig. 3, mercury is sorbed to over 98% efficiency for 60 minutes by a
gold-spiked alumina sorbent, and these beads are repeatedly regenerated by
heating to 600°F. In Fig. 4, mercury is sorbed to 99% efficiency for 80
minutes by a ceramic filter into which sorbent has been imbibed, and the filter
is repeatedly regenerated by heating to 700°F.
FIELD TESTING AT PLASMA HEARTH THERMAL TREATMENT UNIT
The Mercu-RE control technology was tested at a pilot-scale thermal
treatment facility being developed by the Department of Energy. This treatment
system is intended to reduce the volumes of hazardous wastes currently
containerized and stored at DOE facilities. Large amounts of mercury are
frequently found in these stored wastes, necessitating mercury control and
measurement for the proper disposal of these wastes.
The thermal treatment system consists of a plasma hearth chamber, a
secondary combustor, an acid gas scrubber, a baghouse, and a bank of HEAP
filters. Figure 5 shows that the mercury concentrations at the inlet to the
sorbent beds were in the range of 10,000 µg/m3 to 60,000 µg/m3,
and that the inlet concentrations were much higher than the outlet
concentrations. Typical mercury removal efficiencies exceeded 99%. Figure 6
shows concentrations of total and elemental mercury leaving the scrubber over a
4-hour period of time. The "spikes" in concentration correlate to the
input of wastes to the thermal treatment unit. The difference in mercury
readings between the total and elemental measurements is the concentration of
speciated mercury (i.e., the concentration of chemically combined mercury.) The
measurement of the mercury concentrations were made with ADA's continuous,
speciating mercury analyzer. This analyzer measures the concentration of
elemental mercury and of all other mercury-containing compounds simultaneously
and in real-time, down to concentrations of 0.2 µg/m3 (Roberts,
et al., 1996). The analyzer responded rapidly to changes in mercury
concentration. The surrogate waste was fed by inserting a can into the hearth
approximately every 20 minutes, resulting in a pulse of mercury in the flue gas.
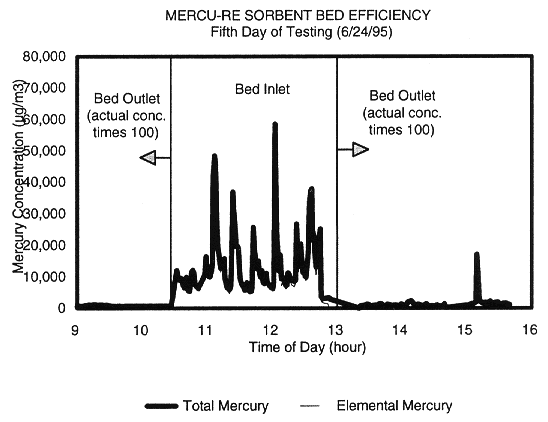
Fig. 5. Sorbent bed removing mercury
from plasma hearth off-gas.
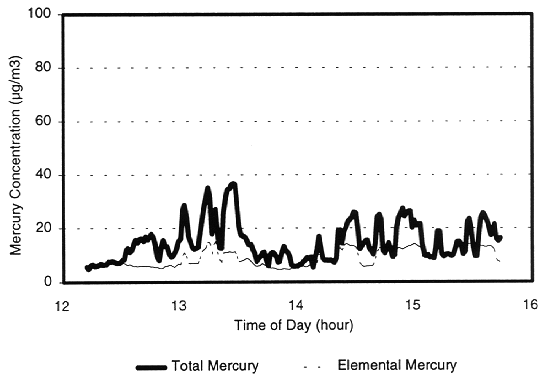
Fig. 6. Expanded view of outlet
concentrations during plasma hearth test.
During the field test, several measurements were made of mercury
concentrations using the Mercury Speciation Sampling Train (MSST) and Draft EPA
Method 29. These sample trains were used to determine the concentration of
mercury in the uncontrolled flue gas. Mass balance calculations (based on
analysis of the feed material and system flue gas flow rates) predicted that the
average mercury concentration would be over 10,000 µg/m3. To
measure these high concentrations with the CEM, a dilution probe was used to
reduce the mercury concentrations by approximately two orders of magnitude. The
analyzer readings verified the mass balance predictions. The range of
concentrations measured using the analyzer (11,300 to 18,100 µg/m3)
agree well with the MSST samples (14,100 to 19,000 µg/m3).
Results using Draft Method 29 were well below analyzer results, ranging from
3,700 to 8,600 µg/m3. It is suspected that the Method 29
impinger solutions may have been overloaded given the high mercury
concentrations in the flue gas.
THERMAL ENDURANCE TESTING
The crystallites of noble metal dispersed on the microporous sorbent support
can, in theory, coagulate by surface diffusion and, in time, compromise the
performance of the sorbent. For this reason, we have initiated an accelerated
thermal endurance test. The test consists of keeping the sorbent at the
regeneration temperature (700°F) for an extended period of time and
periodically removing samples to determine the average crystallite size. This
test is an accelerated test because in commercial application, the sorbent would
be at the regeneration temperature only approximately four hours per day. The
crystallite size measurement is made with x-ray diffraction, specifically
looking at the line broadening that occurs when the crystallites are smaller
than about 100 nm (Cullity, 1978).
We have exposed three sorbents to the regeneration temperature for 90 days,
representing 540 days of commercial operation. So far, one of the three sorbents
is quite stable, one is approaching a stable crystallite size, and the other
needs more data to be sure that it is stable (Fig. 7). The thermal stability of
the sorbent appears to be sufficient for commercial operation.
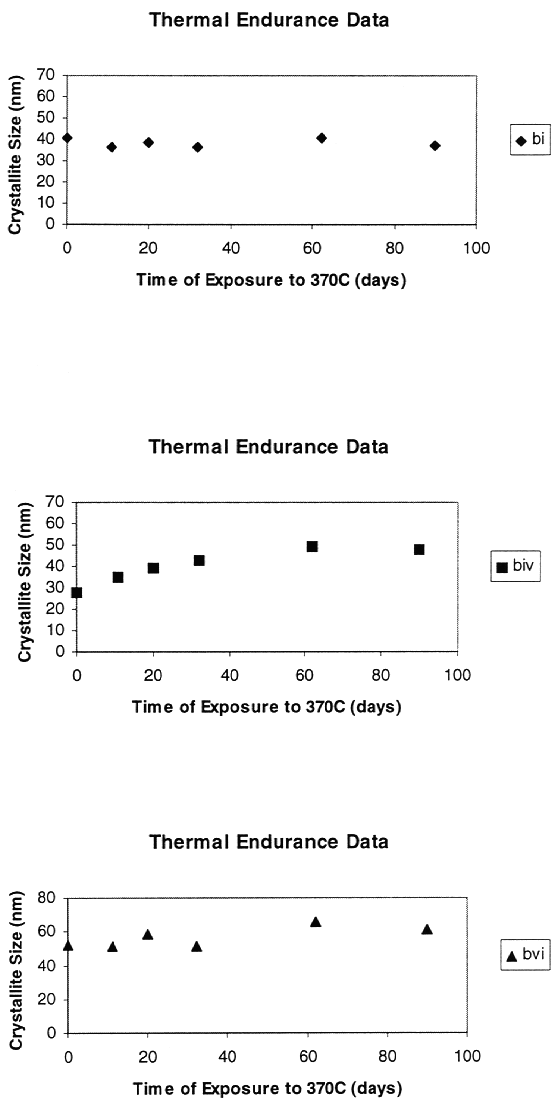
Fig. 7. Stability of noble metal
during exposure to high temperature.
FUTURE PLANS
Thermal endurance itself is only the beginning of the life time challenges
the sorbent must face. The repeatable uptake of mercury` through 100 cycles in
the laboratory is the next test planned. Assuming that there is one or more
sorbents that passes this test with less than 10% degradation in performance, we
will then test the sorbent for 100 cycles in the field on off-gas from a plasma
hearth facility. In this test, the sorbent will be exposed to potentially
condensable poisoning agents such as arsenic, selenium, or vanadium. Other
aggressive components will be present also such as NOx, SO2,
HC1, and water vapor. These tests are planned for Fall, 1997.
BIBLIOGRAPHY
CULLITY, B. D., Elements of X-Ray Diffraction, Addison-Wiley Publishing
Company, Inc., 1978.
DUMAREY, R., R. DAMS, J. HOSTE, "Comparison of the collection and
Desorption Efficiency of Activated Charcoal, Silver, and Gold for Determination
of Vapor-Phase Atmospheric Mercury," Anal. Chem., 57, 2638-43 (1985).
HADEISHI, T., D. A. CHURCH, R. D. MCLAUGHLIN, B. D. ZAK, M. NAKAMURA, B.
CHANG, "Mercury Monitor for Ambient Air," Science, 187,348-9 ( 1971 ).
KVARTEK, E. J., W. H. CARLTON, M. DENHAM, L. ELDRIDGE, N. C. NEWMAN, "Assessment
of Mercury in the Savannah River Site Environment," WSRC-TR-94-0218ET,
September, 1994.
PERONA, J. J., C. H. BROWN, "A Technology Assessment for
Mercury-Containing Mixed Wastes," DOE/MWIP-9, Oak Ridge National
Laboratory, March, 1993.
ROBERTS, D. L., R. W. MARMARO, R. J. SCHLAGER, "Continuous Emissions
Monitor for Total, Elemental, and Total Speciated Mercury," presented at
EPRI-DOE-EPA Joint Workshop on Mercury Measurement and Speciation Methods for
Utility Flue Gas, Research Triangle Park, NC, January 24-25, 1996.
SLEMR, F., W. SEILER, C. EBERLING, P. ROGGENDORF, "The Determination of
Total Gaseous Mercury in Air at Background Levels," Anal. Chim. Acta, 110,
35-47 (1979).
TAYLOR, P. A., K. T. KLASSON, S. L. CORDER, "Mercury Separation from
Aqueous Wastes," presented at the Summer National Meeting of the American
Institute of Chemical Engineers, July 30-August 2, 1995.
TURNER, R. R., C. R. OLSEN, W. J. WILCOX, "Environmental Fate of
Mercury and Cesium-137 Discharged from Oak Ridge Facilities," presented at
18th Annual Conference on Trace Substances in Environmental Health, Columbia,
MO, June 4, 1984.






