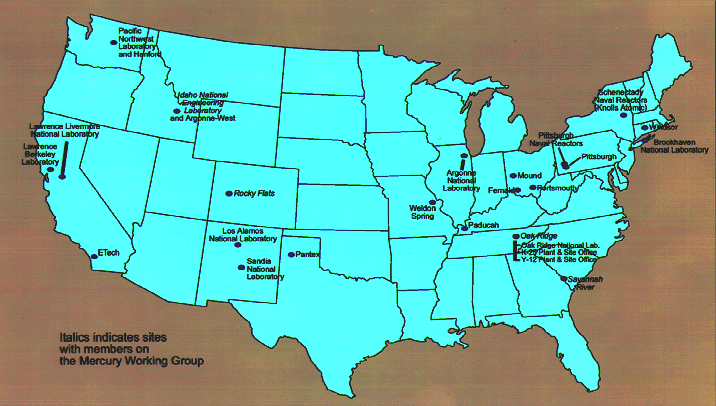
Fig. 1. United States Department of
Energy facilities with mercury-contaminated mixed wastes.
Thomas B. Conley* and Michael I. Morris
Chemical Technology Division
Oak Ridge National Laboratory**
P. O. Box 2008
Oak Ridge, Tennessee 37831-6202
Heather Holmes-Burns
Savannah River Technology Center
Westinghouse Savannah River Company
P. O. Box 616
Aiken, South
Carolina 29802
Jeff Petersell
AIMS, Inc.
Rocky Flats Plant
P.O. Box 464, Bldg. 881
Golden, Colorado 80402-0464
Lynn Schwendiman
Lockheed Martin Idaho Technologies
Company
P. O. Box 1625, MS-8102
Idaho Falls, Idaho 83415
ABSTRACT
In May 1996, the U.S. Department of Energy (DOE) Mixed Waste Focus Area (MWFA) initiated the Mercury Working Group (HgWG), which was established to address and resolve the issues associated with mercury-contaminated mixed wastes. Three of the first four technology deficiencies identified during the MWFA technical baseline development process were related to mercury amalgamation, stabilization, and separation/removal. The HgWG will assist the MWFA in soliciting, identifying, initiating, and managing all the efforts required to address these deficiencies.
The focus of the HgWG is to better establish the mercury-related treatment needs at the DOE sites, refine the MWFA technical baseline as it relates to mercury treatment, and make recommendations to the MWFA on how to most effectively address these needs. The team will initially focus on the sites with the most mercury- contaminated mixed wastes, whose representatives comprise the HgWG. However, the group will also work with the sites with less inventory to maximize the effectiveness of these efforts in addressing the mercury- related needs throughout the entire complex.
INTRODUCTION
Mercury, in various elemental and speciated forms, is present in numerous DOE mixed waste streams. Over 38,000 m3 of mixed low-level and transuranic waste containing mercury have been identified in the DOE complex (see Fig. 1 for locations). Traditionally, mercury has been one of the most difficult contaminants to stabilize in hazardous or mixed waste. Portland cement does not directly stabilize either elemental mercury or mercury salts, and high- temperature techniques such as incineration and vitrification volatilize the mercury, producing off-gases that are dangerous to workers.

Fig. 1. United States Department of
Energy facilities with mercury-contaminated mixed wastes.
In 40 CFR 268.40, the Resource Conservation and Recovery Act (RCRA) defines several categories of mercury wastes, each of which has a defined technology-based treatment standard, or a Universal Treatment Standard (UTS). For nonwastewaters with mercury contaminant concentrations greater than or equal to 260 mg/kg and RCRA-regulated organic contaminants (other than incinerator residues), incineration or retorting (IMERC or RMERC) is identified as the treatment standard. For nonwastewaters with mercury contaminant concentrations greater than or equal to 260 mg/kg that are inorganic, including incinerator and retort residues, RMERC is the identified treatment standard. Amalgamation (AMLGM) is identified as the treatment standard for elemental mercury; however, mercury condensates from RMERC processes will also require amalgamation. Additionally, residues from IMERC processes with greater than 260 mg/kg of mercury contamination will require RMERC, followed by AMLGM of the condensate residues. IMERC residues with less than 260 mg/kg will also require some form of stabilization (i.e., SPC) to meet the RCRA Toxicity Characteristic Leaching Procedure (TCLP) limit for mercury of 0.20 mg/L. This procedure is described in Method 1311 of U.S. Environmental Protection Agency (EPA) Publication SW-846. Nonwastewaters with mercury concentrations less than 260 mg/kg must simply meet the TCLP limit for mercury of 0.20 mg/L.
MERCURY TREATMENT RELATED DEFICIENCIES
During the initial phases of its formation, the MWFA sent representatives to the DOE sites to ask specific questions related to their needs, capabilities, and program status. These questions, referred to as"Standard Questions for Site Visits," were developed prior to the site visits. The purpose of identifying these standard questions was to ensure that the data required to establish a defensible technical baseline were collected to the greatest extent practical.
Upon completion of each site visit, a trip report was generated that documented the information that had been gathered. These reports, which were generally completed within a few weeks of the site visit, include the identified priority needs, potential "quick wins", and status of the technology development activities at that site. The information contained in the trip reports and an initial needs assessment was incorporated into the MWFA Technical Baseline Report (DOE/ID-10524). Some needs defined specific technology gaps in the treatment systems identified for the waste types, while others are general system needs. The technology development needs and potential quick wins compiled in DOE/ID-10524 provide the customer-defined justification for the technical baseline. Through this effort, the MWFA has identified mercury control as a common deficiency throughout the complex. As such, there are several distinct mercury issues , which have been identified in the following areas: mercury amalgamation, mercury stabilization, mercury removal, mercury monitoring, and mercury filtration.
Historically, mercury amalgamation has been used to extract precious metals (i.e., gold, silver) from the materials present. For example, gold is amalgamated with mercury and extracted from a material matrix. The amalgam is then retorted to volatilize the mercury and recover the gold. The definition of amalgamation provided in RCRA 40 CFR 268.42, Table I, indicates that the primary purpose of the process is to reduce the emission of elemental mercury vapors into the air. In addition, the defined leachability limit must also be met, through adherence to the UTS or a defined technology-based treatment standard. The only commercial amalgamation process that shares these waste form requirements is the production of dental amalgams for tooth restoration. Unfortunately, this type of process is only performed on a very small scale, generally using a mortar and pestle to develop the amalgam.
Several treatability studies and other developmental efforts have been conducted throughout the DOE complex related to the amalgamation of mercury wastes. (1,2,5) However, these studies have been performed at a bench-scale level only. Consequently, the primary deficiency with amalgamation of mixed wastes is related to scale-up of the process to a cost-effective operations level. Successful amalgamation is based on several parameters (1) that are dependent on the size of the operation, are themselves interdependent, and will have to be optimized during the scale-up process. In addition, these parameters may or may not be applicable, depending on the basic amalgamation process chosen (i.e., sulfur, copper, SPC). They can be summarized as follows:
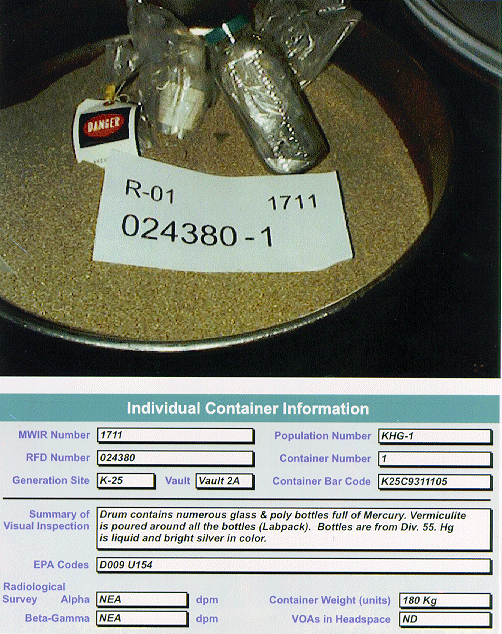
Fig. 2. Example of elemental mercury
waste with cocontaminants.
Mixed wastes containing mercury at concentrations less than 260 ppm are not required to undergo any mercury removal or separation steps; however, the final waste form must leach no more than 0.2 mg/L of mercury as measured by the TCLP. Many mercury-bearing DOE wastes are in the form of debris, non-aqueous sludges, adsorbed liquids, or partially or fully stabilized sludges. Mercury-contaminated soils are also of significant concern. Mercury contained in debris, soils, and stabilized sludges is not readily accessible to leaching agents or thermal desorption and the successful removal of mercury from such wastes has not been demonstrated. The mercury may be chemically bound to a matrix constituent such as vermiculite, portland cement, or clay; alternatively, it may be physically trapped in the matrix, but still leachable in excess of the prescribed TCLP limits. Thus, it may be more practical to stabilize some mixed wastes to comply with regulations rather than extracting the mercury from them.
With the exception of mercury amalgamation, no industrial standard has been identified for the broad application to mercury stabilization in a wide variety of waste matrices. However, conversion of mercury to its sulfide salt is commonly recognized as a stable, essentially inert form. Sulfur polymer cement has been proposed but may not adequately penetrate some matrices. Commercial proprietary processes are marketed for soils, but general applications may be limited.
Improved mercury stabilization processes would chemically or physically bind mercury to meet TCLP limits for the complex sludges, stabilized wastes, soils and debris in the DOE inventory. Processing protocols must ensure adequate stabilization, as well as include measuring and monitoring methods to control and verify the process. Proposed technologies should include systems to minimize worker exposure and secondary waste generation, while maximizing operational flexibility and radionuclide containment. Some of the performance requirements for mercury stabilization are as follows (3):
Mercury separations technologies must deal with an unfortunate condition of many DOE wastes; that is, they contain other contaminants that must be treated in addition to the mercury. The presence of mercury complicates the design of the off-gas systems, the stabilization of residuals, and the monitoring of effluents. In addition to the regulatory drivers for mercury removal in wastes contaminated above 260 ppm, it may be advantageous from a processing/operational viewpoint to remove mercury as a pretreatment step in order to simplify the treatment operations. Some of the primary functional performance requirements for mercury separations processes are as follows: (4)
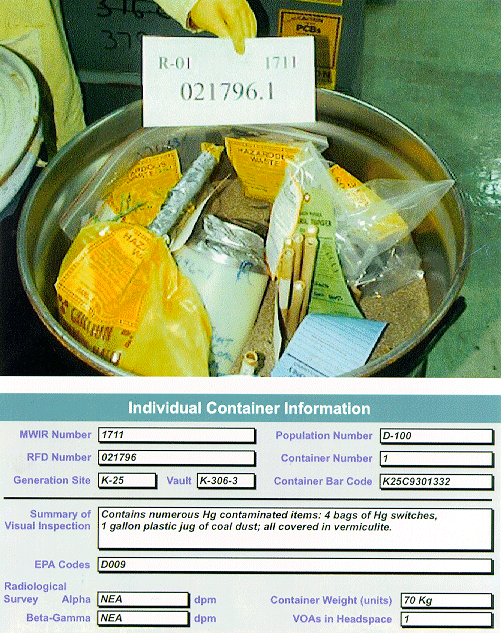
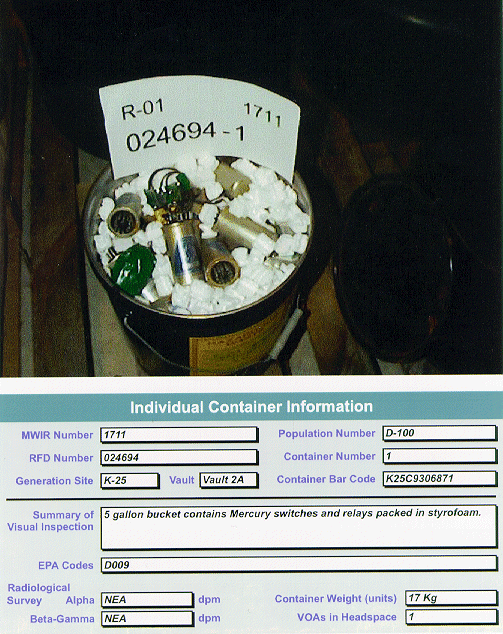
FORMATION OF THE HGWG
In February 1996, the DOE Oak Ridge Operations Office (DOE-ORO) submitted a response to the MWFA's Request for Information in Support of the Quick Wins for FY 1996. In that submission, DOE-ORO proposed the formation of a Mercury Working Group (HgWG). The HgWG was to support the Waste Type Manager (WTM) for "Unique Wastes," which include mercury-contaminated wastes. In addition, it would assist the WTM and, thus, the MWFA in setting the strategy and coordinating all technology development activities related to mercury-contaminated wastes, including work initiated outside the HgWG efforts (i.e., MWFA Calls for Proposals to DOE and Morgantown Energy Technology Center research). The Oak Ridge Reservation (ORR) was established as the lead organization for the HgWG in May 1996, based on the facts that 1) the ORR has the largest inventory of mercury-contaminated mixed waste in the DOE complex, and 2) the ORR has developed a significant technology and science base relevant to mercury that has led to a thorough understanding of the unique problems associated with these wastes throughout the DOE complex, providing the ability to adopt an integrated systems approach to solutions. The ORR member of the HgWG will serve as the HgWG chair.
The focus of the HgWG is to better establish the mercury related treatment needs at the DOE sites, refine the MWFA technical baseline as it relates to mercury treatment, and make recommendations to the MWFA on how to most effectively address these needs. Toward that end, HgWG membership has been established with representatives from sites with the largest mercury-contaminated mixed waste inventories. These sites include OR, Savannah River Site (SRS), Rocky Flats Environmental Technology Site (RFETS), and Idaho National Engineering Laboratory (INEL) (see sites in italics on Fig. 1).
HGWG APPROACH AND PLANS
The first task of the HgWG is to develop a thorough understanding of the physical and chemical characteristics of the mercury-contaminated mixed wastes throughout the DOE complex. In addition, the planned treatment for these wastes must be understood, based on the appropriate Federal Facility Compliance Act (FFCA) Consent Order. The HgWG will also work with site representatives to identify/ understand the perceived technology development needs for each site. The team will initially focus on the sites with the most mercury-contaminated mixed wastes, whose representatives comprise the HgWG. However, the group will also work with the sites with less inventory to maximize the effectiveness of these efforts in addressing the mercury-related needs throughout the complex.
Armed with a more thorough understanding of the DOE complex needs, the MWFA, through the HgWG, will utilize two primary mechanisms to begin addressing these needs: 1) the Request for Proposals (RFP) to industry for mercury amalgamation, stabilization, and separation/removal, and 2) the Call for Proposals (CFP) to DOE for mercury separation/removal. The CFP was issued in July 1996 and responses are currently being reviewed and evaluated. A Commerce Business Daily announcement was published by the HgWG in July 1996, soliciting interest from the private sector for participation in the upcoming RFP. To date, almost 50 expressions of interest have been received by the HgWG.
This course of action was determined, based on the responses received to a Request for Information (RFI) related to the three areas of mercury needs. The results from the RFI indicated that the technical bases exist in private industry to treat, at or near the production scale, those mercury-contaminated wastes that would require stabilization or amalgamation. Demonstrations of specific related technologies will provide the venue through which those technical bases would be applied to the unique problems associated with mercury-contaminated mixed wastes throughout the DOE complex. Demonstrations in these two areas will be identified and performed, beginning in FY 1997, at a sufficiently large scale to assist smooth, timely transition of the successful processes to production readiness for implementation as available treatment systems for applicable DOE wastes.
Additionally, the RFI results indicated that mercury separation/removal is a technology area that still requires the efforts of the research and development community within both DOE and the private sector. Consequently, in addition to the RFP, a CFP for mercury separation/removal technologies was issued in July 1996. This CFP will identify demonstrations for initiation in FY 1997.
The procurement actions associated with these demonstrations and the coordination with the affected sites will be the responsibility of the HgWG. Additionally, research and development activities that are initiated through other efforts that will have an impact on meeting DOE complex needs related to mercury- contaminated mixed waste will also be administered and coordinated for the MWFA by the HgWG. This will allow the MWFA to have all mercury-related technology development activities managed through one central point, ensuring that the deficiencies are adequately defined, the needs are effectively addressed. All duplicative efforts are eliminated, and DOE sites can attain full regulatory compliance relative to mercury- contaminated mixed wastes.
REFERENCES
*Author to whom questions should be addressed.
**Managed by Lockheed Martin Energy Research Corp. for the U.S. Department of Energy under contract DE-AC05-96OR22464.