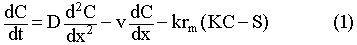RATE LIMITED ISOTOPIC EXCHANGE INVOLVING NATURAL URANIUM
IN THE APACHE LEAP TUFF
Ernest Hardin and Randy Bassett
The University of
Arizona
Michael Murrell
Los Alamos National Laboratory
ABSTRACT
Waterrock interaction involving uranium was investigated by isotopic
analysis of 238Useries nuclides in pore water and secondary
minerals. Samples of secondary U were obtained by selectively leaching intact
rock samples, and by digestion of fracture lining minerals, from the Apache Leap
Tuff. Leaching was used in order to preserve the structure of the waterrock
interface. Leachates analyzed by mass spectrometry with isotope dilution, were
found to contain much more U than present in pore water, yet with similar 234U/238U
activity ratios. In principle these observations could have resulted solely from
U coprecipitation with secondary minerals such as manganese oxides, but a model
incorporating isotopic exchange provides a plausible alternative. Both
coprecipitation and isotopic exchange hypotheses lead to predictions of U
retardation in situ, but isotopic exchange can operate continuously without
requiring transport and precipitation of a carrier species.
Isotopic exchange between groundwater and a high affinity sorbent layer was
treated analytically as retarded diffusive transport within the layer, and
equated with a classical firstorder kinetic model to derive a rate
constant representing layer diffusion behavior. Firstorder sorption rate
parameters could then be calculated directly from laboratory selective leaching
data, indicating that waterrock interaction is rate limited on the time
scale of 234U decay (t1/2 = 245,000 yr). Similar results
were obtained for intact core samples spanning the vadose zone at Apache Leap,
representing a range of hydrologic conditions. These parameters can be used to
compute rate dependent solute retardation in the rock matrix in situ.
The layer diffusion model was also applied to UTh analysis of fracturelining
manganese oxide minerals from the Apache Leap Tuff. The 234U/230Th
systematics indicated opensystem behavior, and the shift was consistent
with isotopic exchange involving 234U. The fracturelining
mineral samples were relatively thick, and the calculated rate constant values
were small. This outcome was predicted qualitatively by the layer diffusion
model, and shows that retardation involving high affinity sorbents encountered
as fracturelining minerals at Yucca Mountain and elsewhere is strongly
rate limited.
BACKGROUND
The 234U/238U activity ratio (AR) in pore waters of
the Apache Leap Tuff increases from about 1.4 in runoff to > 6 in perched
water (Fig. 1). Fractionation is correlated with an oxidative weathering
horizon, and is apparently caused by the autooxidative selective leaching
mechanism of Petit et al. (1). Manganeseoxides contain much of the
secondary U present in this tuff, as shown from leaching studies on crushed rock
(2). A corehole was airdrilled through the vadose zone, intercepting a
perched water table at approximately 150 m depth. Core samples were analyzed for
chemical composition, hydraulic and geophysical properties. Core was preserved
in airtight packages, and later sampled for environmental isotopes by core
squeezing and distillation (3). Intact core samples were leached with MilliQ
water, then a solution of 0.1 M hydroxylamine hydrochloride and 3% acetic acid,
to sample the U abundance and 234U fractionation in pore water and
secondary manganese oxides. Leaching with hydroxylamine produced U in quantities
far exceeding the U dissolved in pore water, yet with similar 234U/238U
activity ratios (Fig. 2).

Fig. 1. Comparison of 234U/238U
activity ratios for waters from the Apache Leap Tuff, sampled by different
methods.

Fig. 2. Uranium mass and 234U/238U
activity ratio for early and late leaching splits, obtained using hydroxylamine,
for several core samples spanning the vadose zone.
The hydroxylamine leaching results can be interpreted to represent either
isotopic exchange or coprecipitation. Apparent 234U/238U
ages for the leached precipitates were calculated assuming: 1) congruent
leaching; 2) minerals sampled by leaching were precipitated from pore waters
with AR equal to that presently observed; and 3) minerals sampled by leaching
were precipitated over a brief time period relative to their 234U/238U
ages ("event precipitates"). The resulting apparent ages are shown in
Table I.
Table I Apparent Age of Precipitates Sampled by Leaching of Intact
Core with Hydroxylamine
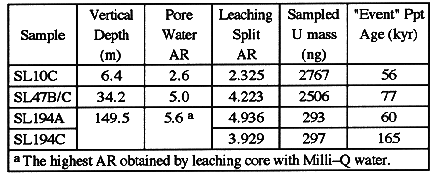
Similar apparent ages are obtained for samples from throughout the unit, and
they are substantially less than the 246kyr half life of 234U.
This similarity is the first line of evidence for isotopic exchange as a
significant waterrock interaction process. Samples listed in Table I
represent a wide range of hydrologic and chemical weathering conditions, yet the
apparent ages are quite recent compared to the formation age (4). To the extent
that precipitation is a continuous rather than "event" process, the
apparent ages would be even less.
TRACTABLE FIRST ORDER MODEL FOR ISOTOPIC EXCHANGE
Waterrock interaction involving a highaffinity sorbent is
modeled in terms of rate limited transport of U species within the sorbent. This
layer diffusion model is equated mathematically to a firstorder kinetic
model, using termwise comparison of series solutions (2). In this way the
macroscopic effect of the entire layer on solute concentration in the mobile
phase is described directly by a rate constant k, and a distribution coefficient
K. These parameters can be used in a classical transport equation:
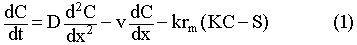
where D is a dispersion coefficient, v is the advective velocity, S is the
immobile phase concentration, and rm is the rockwater mass
ratio.
The observable quantity from which k and K can be deduced is the composite
AR of secondary mineral precipitates, which can be sampled directly from
fracturelining minerals, or sampled by leaching intact core. Although the
model derivation shows that the effective rate constant for the entire layer is
inversely proportional to the square of the layer thickness, it is not necessary
to know the layer thickness in order to quantify k and K. The steady state AR of
U dissolved in fracture water or matrix pore water is required for the
calculation.
OBSERVATIONS INTERPRETED USING THE ISOTOPIC
EXCHANGE/LAYER DIFFUSION MODEL
Using the leaching data of Table I, the matrix sorption parameters in Table
II were calculated using an iterative scheme (2). Leaching may be incomplete, so
the proportion of the sorbent layer dissolved congruently by leaching (p) is a
variable in the problem. The U yield from leaching is known, so p is
proportional to the whole rock distribution coefficient assuming that the pore
water and the sorbent are in chemical equilibrium. Knowing the AR for two
successive leaching splits from the same sample, and the pore water AR, it is
possible to solve for the three parameters k, K, and p. As an example
calculation, sorption parameters from Table II were used in a lumped parameter
model to compute U retardation (Fig. 3).
Table II Sorption rate constant and distribution.
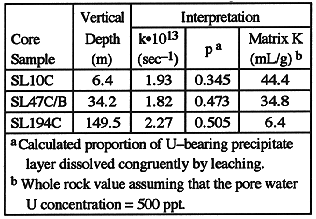

Fig. 3. Hypothetical curves for
uranium transport through tuff matrix, computed using sorption parameters
interpreted from laboratory leaching, and a lumped parameter model with
residence times as indicated.
The isotopic exchange/layer diffusion model was also used to modify the
classical Useries relationship describing 230Th/234U/238U
behavior in a mineral precipitated from pore water with known AR. The closed
system "concordia" curve shown in Fig. 4 depicts the initial
coprecipitation of fractionated U from solution without Th, which is insoluble.
The initial 234U/238U activity ratio is greater than
unity, and 230Th/238U is zero. As the precipitate ages
there is ingrowth of 230Th, and the excess 234U decays
toward secular equilibrium. Isotopic exchange causes the curves to shift,
depending on the rate constant as shown. Each curve terminates along the
diagonal line representing dynamic isotopic equilibrium. Loss of 234U
from direct recoil (or selective leaching) causes a different shift in the
curves as shown.

Fig. 4. Modified U-series condordia
plot showing the behavior of 234U and 230Th after
precipitation from a groundwater with known AR, under 234 Urecoil/leaching and
isotopic exchange conditions.
Three samples of fracture lining manganese oxides from Apache Leap were
analyzed for 230Th/234U/238U by high
resolution mass spectrometry, and found to plot along the steady state line in
Fig. 4. This is the second line of evidence for isotopic exchange as a
significant waterrock interaction process. Corresponding values for the
sorption rate constant are smaller than those calculated for matrix sorption in
Table II. The isotopic exchange/layer diffusion model predicts that the
effective rate constant decreases significantly for thicker layers, which is the
case for fracturelining precipitates. Thus although secondary fracture
lining manganese oxide minerals are common in certain tuff units at Yucca
Mountain, particularly hydraulically transmissive units in the saturated zone
such as the Tram Member of the Crater Flat Tuff (5), the contribution of these
phases to U retardation is strongly rate limited.
SUMMARY
Observations of U fractionation in secondary minerals of the Apache Leap
Tuff can be evaluated in terms of coprecipitation or isotopic exchange. The
hypotheses are inclusive, and both lead to predictions of U retardation in situ,
but isotopic exchange does not require transport and precipitation of a carrier
species, and is inherently plausible as a continuously operating retardation
mechanism. Isotopic exchange explains the isotopic similarity of leachates from
samples spanning the vadose zone at Apache Leap. A conceptual and numerical
model of isotopic exchange is proposed based on retarded diffusive transport
within a highaffinity sorbent layer. The model was used to estimate the
rate constant of exchangeable sorption from laboratory leaching of intact rock
samples. The isotopic exchange/layer diffusion model is consistent with isotopic
analyses of fracturelining manganese oxides, which are thicker and exhibit
strongly ratelimited interaction with U in fracture waters.
REFERENCES
- PETIT, J.C., Y. LANGEVIN and J.C. DRAN, 1985, "234U/238U
disequilibrium in nature: theoretical reassessment of the various proposed
models," Bull. Minéral., V.108, pp.745753.
- HARDIN, E. L., 1996, "Uranium234 in Vadose Zone and Perched
Waters of the Apache Leap Tuff, Central Arizona," Ph.D. Dissertation,
Department of Hydrology and Water Resources, The University of Arizona.
- DAVIDSON, G. R., E. L. HARDIN and R. L. BASSETT, 1995, "Extraction of
14C from Pore Water in Unsaturated Rock Using Vacuum Distillation,"
Radiocarbon, V.37, N.3, pp.861874.
- PETERSON, D.W., 1968, "Zoned ashflow sheet in the region around
Superior, Arizona," in: S.R. Titley (ed.), Southern Arizona Guidebook III,
Geol. Soc. America, pp.215223.
- CARLOS, B. A., S. J. CHIPERA, D. L. BISH and S. J. CRAVEN, 1993, "Fracturelining
manganese oxide minerals in silicic tuff, Yucca Mountain, Nevada, U.S.A.,"
Chem. Geol., V.107, pp.4769.