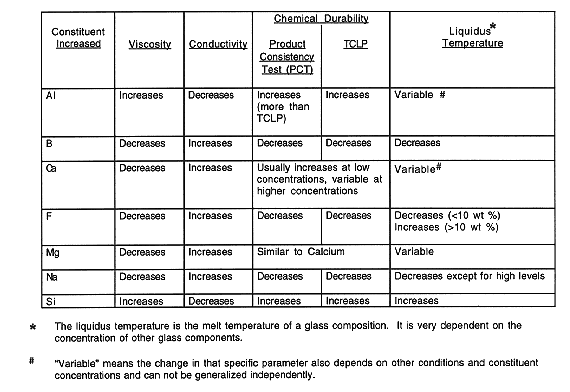
Table I Summary of Effects of Major Glass Constituents on Glass
Properties
Earl W. Holtzscheiter, Sandra J. Carroll
Westinghouse
Savannah River Co.
Aiken, SC 29808
James E. Flaherty
SAIC
20300 Century Blvd.
Germantown, MD 20874
ABSTRACT
The U. S. Department of Energy (DOE) is charged with responsibly managing the largest volume of mixed waste in the United States. This responsibility includes managing waste in compliance with all applicable Federal and State laws and regulations, and in a cost-effective, environmentally responsible manner. Managing certain treated mixed wastes in Resource Conservation and Recovery Act (RCRA) permitted storage and disposal units (specifically those mixed wastes that pose low risks from the hazardous component) is unlikely to provide additional protection to human health and the environment beyond that afforded by managing these wastes in storage and disposal units subject to requirements for radiological control.
In October, 1995, the DOE submitted a regulatory reform proposal to the Environmental Protection Agency (EPA) relating to vitrified mixed waste forms. The technical proposal supports a regulatory strategy that would allow vitrified mixed waste forms treated through a permit or other environmental compliance mechanism to be granted an exemption from RCRA hazardous waste regulation, after treatment, based upon the inherent destruction and immobilization capabilities of vitrification technology. The vitrified waste form will meet, or exceed the performance criteria of the Environmental Assessment (EA) glass that has been accepted as an international standard for immobilizing radioactive waste components and the LDR treatment standards for inorganics and metals for controlling hazardous constituents. The proposal further provides that vitrified mixed waste would be responsibly managed under the Atomic Energy Act (AEA) while reducing overall costs. Full regulatory authority by the EPA or a State would be maintained until an acceptable vitrified mixed waste form, protective of human health and the environment, is produced.
INTRODUCTION
A technical data package has been prepared to support a regulatory strategy which would allow vitrified mixed waste forms which have been treated through an environmental permitting process to be granted an exemption from RCRA hazardous waste regulations based upon the inherent destruction and immobilization capabilities of the technology. Additionally, it proposes an alternative sampling and analysis strategy for certain highly radioactive mixed waste forms which will give the same level of confidence as sampling and analysis of the final waste form. The technical data package was submitted to the Environmental Protection Agency (EPA) in October, 1995 in support of the draft Hazardous Waste Identification Rule (HWIR). This paper is a summary of the Department of Energy's technical data package and their conditional exemption proposal for vitrified mixed waste.
Vitrification is the process of converting materials into a glass or glass-like substance, typically through a thermal process. Vitrification thermally destroys organic contaminants and stabilizes inorganics and metals by incorporating them into the glass structure.
Vitrification has four major advantages over other methods of waste management. The primary advantage is the durable waste glass it produces. With proper formulation, this waste glass performs exceptionally well in leach tests. The second major advantage of vitrification is the ability of the waste glass to incorporate a wide variety of contaminants and accompanying feed material in its structure without a significant decrease in quality. The third advantage is that vitrification processes can accommodate both organic and inorganic contaminants of various amounts. Lastly, vitrification typically results in significant volume reductions of waste material, depending on waste composition (1).
Vitrified waste forms possess an inherent environmental stability and will resist degradation for thousands of years, until radioactive decay lessens the threat to human health and the environment. During this decay period, the metals and inorganics are chemically bonded in the glass matrix. Due to these features, EPA has declared vitrification to be the specified treatment technology for mixed high-level waste (FR 55 22627).
DOE's mixed waste are subject to dual regulation: the hazardous components are managed under RCRA, and the radioactive components are managed under the Atomic Energy Act (AEA). The AEA of 1954, as amended, authorizes the DOE to protect the health and safety of the public against radiation in conducting the Department's programs. Under the authority of the AEA, DOE has established a series of Orders which describe various self-imposed requirements. DOE Order 5820.2A "Radioactive Waste Management" (2), establishes policies and guidance for the management of radioactive waste and contaminated facilities.
Order 5820.2A describes a performance-based approach which allows DOE to utilize a variety of designs based upon local disposal conditions to achieve the performance criteria of protection to human health and the environment that is required under the Order. Under RCRA, mixed waste is subject to prescriptive requirements that govern the design and operation of the disposal facility, while still achieving the same goals as under DOE's Order.
Significant volumes of mixed waste have been generated within the DOE complex from various site production and maintenance activities. Process wastes that are candidates for vitrification are primarily high-level waste sludges and slurries stored at the Hanford Site and the Idaho National Engineering Laboratory (INEL). These wastes are hazardous due to their corrosivity, the presence of toxic metals, and/or the presence of listed chemicals. In addition to high-level wastes, mixed low-level wastes that are predominantly sludges, slurries and metal/metal oxides are also candidates for vitrification.
Distribution and Volume of Waste Streams that are Planned for Vitrification
Eight DOE sites have produced or will produce (over the next 75 years) wastes that are planned to be vitrified and that are potentially impacted by the DOE's Regulatory Reform proposal. They are- Battelle, General Atomics, Knolls, Rocky Flats, the Hanford Site, INEL, the Savannah River Site (SRS) and the Oak Ridge Reservation (ORR). Total existing and projected (75 year) inventory of mixed high-level and low-level wastes that are planned for vitrification at these eight sites is approximately 272,000 cubic meters. Approximately 74% of the waste is high-level and 26% is low-level. The majority of this waste, about 98%, is stored or predicted to be generated at the Hanford Site (3).
Waste Form and Contents
The physical form of wastes planned for vitrification is varied. High-level waste consists predominately of aqueous liquids and inorganic sludges. The high-level waste at INEL has been calcined and consists primarily of heavy metal salts and oxides. (Calcination is the process of transforming high-level waste into a solid non-corrosive granular form using a heated fluidized bed).
Mixed low-level waste matrices that are planned for vitrification include glass debris, waste water treatment sludges, soil, acidic waste waters, inorganic particulates, organic debris, ash, inorganic heterogeneous materials, and predominantly inorganic materials. The largest category for mixed low-level waste, "predominantly inorganic" includes filters, insulation, metallic waste and decontamination and decommissioning wastes. "A second category, inorganic homogeneous" consists primarily of sludges and particulates.
Vitrification Process Description
Vitrification is the thermal-chemical process whereby oxides of elemental constituents are incorporated into a solid, continuous, non-crystalline, three-dimensional network or glass structure. Glass is further defined as an inorganic product of thermal fusion that has cooled to a rigid condition without crystallizing (3). Vitrification, which occurs in a liquid mixture at an elevated temperature (nominally 1000°C to 1500°C), chemically bonds the glass elemental constituents together using oxygen to form a solution. At the required operating temperatures, organic components are either destroyed or volatilized and decompose, with decomposition products captured in the off-gas treatment system rather than being incorporated into the glass product. At least one of the glass-forming elemental oxides, termed "network formers," must be present in the liquid mixture in sufficient quantity to form the glass matrix as the molten solution cools. The four primary network former oxides are those of silicon, phosphorous, boron, and germanium. Other elements break the glass-forming bonds and can lower the melt viscosity or produce other changes in the glass physical characteristics. These oxides are "network modifiers" and include the alkali metal and alkaline earth oxides. Some elements can replace to a limited extent the glass-formers in the glass matrix and are called "intermediate" oxides. These oxides include those of aluminum, vanadium, titanium, beryllium, molybdenum, bismuth, iron, zirconium, and others (1). Because the network modifiers can greatly influence glass properties such as durability, viscosity, strength, and leach resistance, glass formulation development balances physical properties with processing requirements.
Most waste glasses are based on the silica network because of the combination of good processing and physical characteristics. However, this requires most hazardous waste to be mixed with silica and other glass formers that are necessary for proper glass formulation. Waste glasses range from approximately 30 wt% actual waste to much higher when the waste itself contains substantial glass formers (e.g. contaminated soils and sludges). Due to the nature of the glass-making process, substantial volume reductions are common. Gases such as radon and nitrogen are maintained in the relatively open glass network and are not chemically bonded like the metal oxides that form the glass structure. Exceptions are fluorine and oxygen. The latter is the bonding element in glass and is a necessary part of the glass structure. Fluorine can be incorporated into some silicate glasses up to ten wt% or more.
Process Limitations
Most elements can be vitrified and incorporated into glass to some extent, however some of the more volatile elements (such as cesium and the halogens) can be incorporated into glass only in very small concentrations (1). Some metals, especially chromium and the noble metals, have limited solubility within many glass melts. However, by proper process control and design even these volatile components can be substantially incorporated into the glass by maintaining a melter cold cap, use of reactive additives, and lower temperature glass formulations (~1050°C).
Many of the waste elements, as well as a high concentration of the network modifiers or intermediates in glass, can cause two-phase or crystalline structure distributions within the glass. Such phases can have substantial impact on the glass properties (1). In homogeneities may also be present in a waste glass due to incomplete melting and homogenization of frit and waste, precipitation of crystallites, phase separation upon cooling of a melt, and devitrification. Any of these processes can cause the final glass to contain separate phases of various sizes and crystallinity. These separate phases and crystalline structures sometimes impact the corrosion and leach resistance behavior of glass. For example, studies of heat-treated Savannah River Laboratory (SRL), waste glasses have found that spinels, nepheline, and acmite devitrification products occupy 1 to 10% of the total glass volume (1). For the Savannah River waste glasses, such devitrification and crystal growth did not detract from the glass leach resistance (1). Crystallization and devitrification can be controlled by vitrification process parameters and final glass cooling controls.
Normally the glass-forming reaction is limited by mass transfer in the melt, so particle size control and good mixing of the solid constituents before melting is beneficial. Otherwise, non-reacted glass-coated chunks of solid material may result. This may be especially important for vitrification of large debris and may require preparation of the debris prior to vitrification. By proper glass formulation, residence time, mixing, and overall process control, durable and acceptable glasses can be consistently produced.
Glass Processing
Table I lists the general effects various elements produce in a silica-based glass composition. Note in general that those components which make the glass more processible (i.e., lower the melt temperature or reduce the melt viscosity) tend to decrease the chemical durability of the glass. The glass durability versus processibility concern is the principal trade-off when waste glass compositions are formulated and tested (1).
Table I Summary of Effects of Major Glass Constituents on Glass
Properties

Destruction of Organic Components
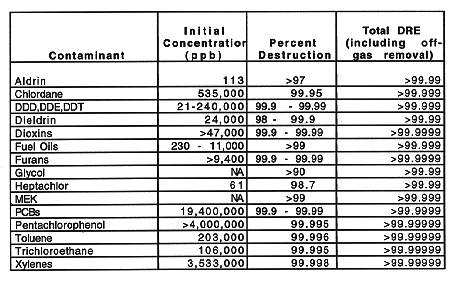
The DOE proposes to eliminate testing requirements for organic constituents identified in waste streams that will be vitrified because organic wastes are thermally destroyed by the vitrification process (vitrification systems typically operate in excess of 1000°C). The destruction occurs through pyrolysis in the melt and combustion in the plenum or in the secondary combustion chamber. Studies have been performed that demonstrate the destruction and removal efficiencies (DREs) for a number of organic contaminants and compounds (1). The DREs listed in Table II are values typical for In Situ Vitrification (ISV) systems. These values are conservative when compared with DREs for conventional melter systems with off-gas components designed to comply with (or exceed) air permit requirements. These DREs meet the requirements of 40 CFR Parts 264 and 265.
Table II In Situ Vitrification Organic Destruction and Removal
Efficiencies (1)
Glass Compositions
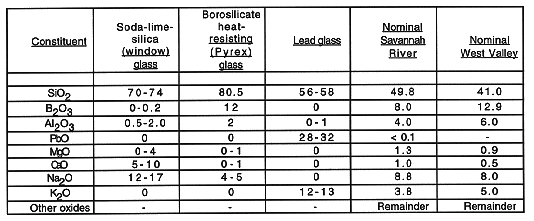
Table III shows several typical commercial glass compositions and the flexibility possible in formulating glass compositions (1). The proposed high-level waste glass for SRS and WVDP are shown for comparison.
Table III Compositions of Silica-based Glasses (wt %)
Environmental Assessment Glass Standard
The DOE proposes to develop vitrified waste forms with glass compositions that possess performance characteristics that meet or exceed the performance standards of the Environmental Assessment (EA) glass formulated as the standard for the immobilization of high-level waste (1). The EA glass is a borosilicate glass. The utilization of borosilicate glass as a waste form is supported by waste form evaluation programs in the United States and other countries. Essentially all nations now reprocessing or planning to reprocess spent nuclear fuels are either using borosilicate glass or have selected it as the preferred waste form for high-level radioactive waste. The EA glass was specifically formulated to retard the release of radioactive components in the waste. In addition, it has been shown that the EA glass retards the release of non-radioactive components. The glass has been subjected to TCLP testing for RCRA metals and the leachate concentration of these metals is well below the concentrations that EPA has proposed as generic exemption levels in the HWIR (1).
Glass Leaching
DOE will use two forms of leach tests to determine the durability of the glass (ability of a glass to retain both the radioactive and RCRA constituents in the matrix). These two types of tests are the Product Consistency Test (PCT) ASTM-C1285-94 or comparable durability leach tests, and the Toxicity Characteristic Leaching Procedure (TCLP). The TCLP test will be used to determine the leach rates for RCRA metal constituents. The PCT was developed for evaluating the performance of high-level waste glass and will be used to determine the durability of the glass as it relates to the release of radioactive components. The durability is determined by monitoring the leach rates of several of the most leachable glass components.
Proposed Testing for Hazardous Metal Constituents
The DOE proposes to pursue leach rate testing for hazardous metal constituents identified in the waste under an approach that considers the radiological hazard associated with testing of the final product and sampling/analytical logistics:
Process Control Program
A vitrification process that will consistently provide an acceptable product requires a process control protocol for key operating parameters that yields a glass product that consistently falls within a pre-defined acceptable performance envelope. The glass formulation is defined by performing treatability studies on either the actual radioactive waste or an appropriate surrogate. The treatability studies provide a glass formulation that will immobilize both radioactive and hazardous components. The glass formulation process includes balancing operating variables such as viscosity with durability and waste loading. Once the right balance of variables is made to allow the production of a durable and processible glass, the range on the operating and glass composition parameters defines the performance envelope. The process control strategy is how the process is run to consistently yield the desired glass formulation (i.e. operate the process within the performance envelope). Once the process control strategy is defined and accepted as part of an environmental permitting process, the inherent properties of the final glass waste form justify no further RCRA Subtitle C control.
Criteria For a Mixed and Hazardous Waste Process Control Program
Overall Process Flowchart and Process Control Points
Prior to the development of the process flow sheet and the equipment and process design, glass treatability studies are performed with either representative surrogates or the real waste depending on the radioactivity levels. In the treatability studies, the glass formulations are optimized for durability (measure of the glass capability to retain radioactive and hazardous components) and melter operating parameters (viscosity and liquidus temperature) consistent with melter capability. The durability is determined by standard leaching tests such as the Product Consistency Test (PCT) which uses the leach rates of the more easily leached chemical components for comparison to the EA glass. In the process design phase while undergoing treatability studies, the glass would be evaluated by TCLP testing for hazardous metal constituents. Once the process flow sheet is defined, then the effluents and product compositions are defined and the appropriate process control strategy is put in place. The following provides a description of a typical implementation strategy of the previously outlined "Criteria For A Mixed and Hazardous Waste Process Control Program" depicted in Fig. 1.
a. Feed forward process control systems will require characterization of key glass components prior to feeding the waste and glass formers to the melter. The acceptance of melter feed will be based on the chemical composition or other means of product quality prediction and key operating parameters (viscosity and liquidus temperature) required to successfully operate the melter.
Note: The chemical composition of the melter feed must be previously determined by either surrogate or real waste testing that can be correlated with waste glass performance as determined by Product Consistency Testing (PCT) or by comparable durability leach tests.
b. Feed back process control systems will typically sample and analyze the product stream (in this case the glass pour stream). The results of the analyzes (either based on leach performance or another process variable correlated with leach performance) will determine whether melter feed is compositionally adjusted and/or rework of previously produced glass is required. The sampling frequency is based on the expected uniformity of the feed and the range of compositions within the process acceptance envelope.
a. Feed forward process control will require waste or melter feed compositional knowledge based on samples. The results of the sampling will be compared to the acceptance criteria (composition, waste loading, etc. that can be related to glass durability). The pretreatment or adjustment of melter feed will be based on sample results and will control the ratio of the waste to glass formers. The extent of re-work would be related to the uniformity of the waste feed.
b. Feed back process control would require sampling of the product stream generally as the melt exits the melter. The composition of the glass would be related to the durability or durability testing could be done directly. If the sample results did not meet acceptance requirements, the glass would have to be reworked.
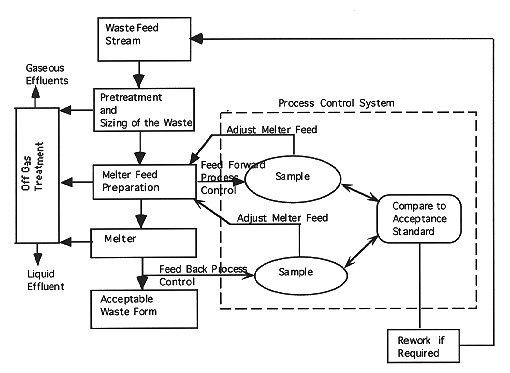
Fig. 1. Typical Mixed and Hazardous
Waste Process Control Flow Chart
REGULATORY CONTROL
DOE'S regulatory reform initiative on vitrified mixed waste requests that EPA acknowledge that the vitrification process provides a glass final product that has inherent characteristics which provide sufficient protection to human health and the environment such that the final form should be granted an exemption from RCRA hazardous waste regulations. In order to produce an acceptable final glass product, the "Criteria for a Mixed and Hazardous Waste Process Control Program" must be followed. Within the context of the permitting process that controls the treatment (through vitrification) of the listed mixed/hazardous waste, the documents necessary to show that the criteria have been met will be provided for review as part of the existing permitting process. In most instances, the treatment of mixed high-level or mixed low-level waste would require a RCRA permit. For example, the RCRA permitting provisions under 40 CFR Part 264, Subpart X, would require the submission of all of the information in the Process Control Program, review and approval of all of those provisions and subsequent issuance of a permit requiring compliance with each component provision. In addition to obtaining a permit for the treatment of waste, most vitrification units will also require an air emissions permit, and a wastewater treatment/effluent permit. Depending upon the actual application and the regulations in the state where the vitrification unit is to be operated, there may be other permitting requirements. In all instances, the regulatory agency retains complete control over the vitrification process and assures, through the permitting/compliance regime, the process produces a glass meeting environmentally acceptable performance characteristics. It is only after the production of glass that meets these performance characteristics (EA glass and LDR treatment standards demonstrated via inorganic analysis are the suggested performance criteria), that DOE is requesting the regulatory authority to allow the waste form to be exempt from RCRA Subtitle C control. The treated waste will continue to be managed in accordance with DOE Order 5820.2A(2).
At the request of some states that may be impacted by a proposed rule to exempt vitrified mixed waste after treatment, the DOE has recently suggested some regulatory language which could be incorporated into existing regulations. This language is subject to input from all interested parties and will likely be further refined as this initaitive moves through the regulatory process. The proposed language developed to date includes an exemption in 40 CFR 261.3 (Definition of Solid Waste) to exclude mixed waste that has been treated by vitrification authorized under 40 CFR Part 270 or another environmental permitting authority accetpable by the Regional Administrator. It includes a provision that terms and conditions can be added through the permitting process to ensure the integrity of the final waste form. The proposed language also includes an addition to 40 CFR 268.40 (LDR Treatment Standards) requiring that vitrified mixed waste meet the LDR treatment standards of Subpart D and that compliance with the treatment standards will be measured by analysis for inorganic constituents listed in the universal treatment standards. The proposed language does not require strict adherence to the performance standards outlined above, thus allowing for flexibility on the part of the permitting authority to set performance standards on a case by case basis.
PROGRESS AND PATHFORWARD
Since submitting this proposal to EPA in October, 1995, the DOE has continued to work with the EPA to answer questions and define areas that need additional consideration. Several meetings have been held and informal comments have been made by EPA staff responsible for reviewing the proposal. In addition, shortly after the submission of the proposal, DOE began working with the National Governor's Association through an existing avenue that brought together states with an interest in DOE's mixed waste activities. Through requests from this group, DOE has produced both the proposed language mentioned above and a cost saving analysis relating the amount of money to potentially be saved if the proposal is adopted. The DOE has also initiated discussions with the National Association of Attorneys General (NAAG), and the Association of State and Territorial Waste Management Officials (ASTWMO). A Regulatory Reform newsletter outlining this and other regulatory reform initiatives has also been widely distributed. In the late summer of 1996, DOE initiated a series of meetings with key stakeholder states. These meetings were one day long technical meetings where the basis of the vitrification proposal (and another regulatory reform proposal on immobilized mixed debris) were discussed in detail, with ample time for questions from attendees. These meetings were held in Augusta GA, Las Vegas NV, Richland WA, Golden CO, Oak Ridge TN and Los Alamos NM. Attendees included representative of EPA and state regulatory agencies, assistant attorneys general, EPA-HQ, NAAG, and ASTWMO. These meetings were well received with the technical basis for the proposals generally supported by attendees. However, a number of issues, common to most state representatives were identified. DOE has established a working group including state representatives from regulatory agencies and attorney general offices to try to identify and resolve issues that may impede adoption of the vitrification proposal. In summary, the preceding discussion proposes that, based on the inherent characteristics of the vitrification process and stability of the glass waste form, the glass product be exempt from RCRA control. This proposal is contingent on the required use of the Process Control Program described in this document. The Process Control Program provides the description of the key operating variables, the process stream compositions as they relate to the end product quality and the permitted emissions/effluents, the acceptable performance envelope, and the end product durability. This information not only defines the process and ensures final product performance, but is required to demonstrate that the process will meet existing federal and state environmental regulations associated with the operation of a mixed waste treatment facility. Therefore, this proposal provides a means to facilitate the use of a superior technology for responsible waste management while reducing costs and maintaining full regulatory authority until an acceptable vitrified waste form is produced.
REFERENCES