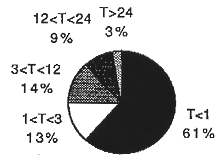
Fig. 1. Distribution of TRU waste
drums in LANL inventory by thermal power ratio (T).
Eugene J. Mroz, Chris Leibman and Stanley T. Kosiewicz
Chemical Science and Technology Division
Los Alamos National Laboratory
Los Alamos, NM 87545
ABSTRACT
More than one-third of the drums in DOEs TRU waste inventory cannot be presently shipped to WIPP in the TRUPACT-II because they exceed the allowed limit of heat generation by virtue of radioactive decay. This limit was imposed to ensure that the amount of hydrogen generated by radiolysis does not achieve the lower explosive limit of hydrogen in air (5% v/v). Los Alamos is working to justify increasing these wattage limits: 1) by demonstrating that the phenomenon of matrix depletion greatly reduces the potential for hydrogen generation and 2) by investigating the use hydrogen getters to actively remove hydrogen from the headspace of the waste drums and/or the TRUPACT-II.
INTRODUCTION
At the present time, about 40% waste drums at LANL cannot be shipped to WIPP because they exceed the thermal power (heat from the decay of transuranic nuclides) limits set forth in the TRUPACT II Safety Analysis Report (SAR). These wattage limits are derived from estimates of the amount of hydrogen calculated to be generated by radiolysis during the 60-day shipping period. All DOE sites with TRU waste are similarly affected.
Figure 1 shows the percentage distribution of TRU waste drums in the LANL inventory according to their thermal power ratio (T). The thermal power ratio (T) is defined as the thermal power rating of the container, as estimated from inventory information, divided by the present regulatory limit for thermal power for the waste matrix and container type. These estimates assume the TRU wastes are contained in four layers of packaging.
Only those drums with T<1 (~14,400) are presently eligible for transportation to WIPP in the TRUPACT-II (provided they meet all other WIPP waste acceptance criteria). Of the remaining ~9000 drums, ~3000 fall in the range of 1<T<3. An approximately equal number of drums are in the category of 3<T<12. Another ~2000 drums are in the category of 12<T<24. And the remaining 643 drums have T>24.

Fig. 1. Distribution of TRU waste
drums in LANL inventory by thermal power ratio (T).
STRATEGIES FOR DRUMS WITH T>3.
At the present time, the only plausible strategy for drums with T>3 is to remove the waste materials from these drums and re-distribute and repackage the waste among multiple drums such that each resulting drum is within the allowable wattage limit. This process would result in the creation of several thousands of new drums of waste. This is both expensive and antithetical to good waste management practice which strives to minimize waste production.
LANLs strategy for getting drums with T>3 eligible for shipment to WIPP is two-fold:
Hydrogen Generation and Matrix Depletion
A particle of Pu adhering to the surface of waste material is continuously irradiating the material with alpha particles. The energy of the alpha particles is sufficient to cause radiolytic changes in the chemistry of the underlying material. When the material is a hydrogenous substance such as cement, cellulosics or plastics, the radiolysis products include hydrogen gas. The hydrogen is generated within a small volume that is determined by range of alpha particles within that material.
For any given material, the amount of hydrogen generated depends on the energy of the alpha particles. This is called the hydrogen G-value and is expressed in units of molecules of hydrogen produced by 100ev of deposited energy. However, the hydrogen G-value is not dose independent. Because of the very limited range of alpha particles within these materials, the irradiated volume is being continuously depleted of hydrogen as the cumulative deposited energy increases. Consequently, the hydrogen G-value also decreases as the cumulative dose increases. This phenomenon has been observed in several different experiments ( Zerwekh, 1979; Kosiewicz, 1981; Zerwekh et al., 1993; Marshall et al., 1994).
The present-day wattage limits for TRU waste containers and the TRUPACT-II are based on G-values established for newly-generated waste. They do not account for the age of the waste drums presently in the DOE-wide inventory and the consequent reduction in hydrogen generation due to matrix depletion.
The Matrix Depletion Project is a joint effort between Idaho National Engineering Laboratory, Rocky Flats Environmental Technology Site and Los Alamos National Laboratory. This project is measuring realistic, age-dependent hydrogen generation rates from TRU wastes. This information will be the basis for an application to the NRC to increase the allowable wattage limits for waste to be shipped in the TRUPACT II.
Los Alamos is investigating dose-dependent hydrogen generation by both 238Pu and 239Pu that has been deposited on material types typically found in TRU waste drums. The effects of heating and vibration such as can be expected to occur during transportation in the TRUPACT-II are also part of our investigation.
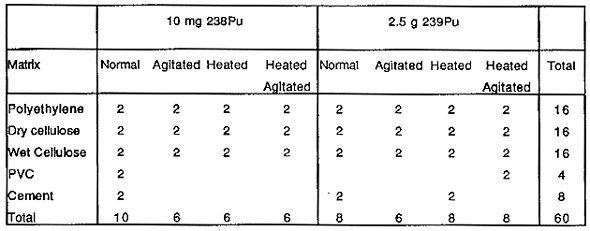
Sixty gas-tight, 1-liter containers were loaded with different waste matrices together with 238Pu and 239Pu oxide powders. The sixty containers are divided among four racks that expose the containers to four different environmental conditions that might be encountered in the TRUPACT-II. These include heating at 60°C and/or agitation that will simulate the expected road vibrations enroute to WIPP. The contents of the 60 containers and the environmental conditions they are exposed to are presented in Table I.
Table I. Test Container Contents for Matrix Depletion Experiments
A diagram of the experimental apparatus is shown in Fig. 2. The headspace gas from each container is sampled and analyzed on an approximately bi-weekly schedule. Hydrogen, oxygen, nitrogen and argon are analyzed by an automated gas chromatography system. The sampling, data acquisition and the gas chromatographic analysis systems all operate under computer control.
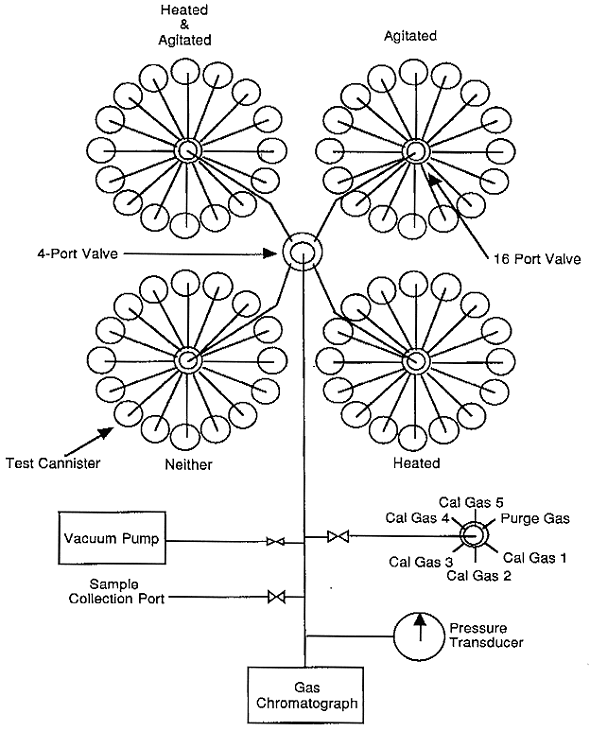
Fig. 2. Diagram of Matrix Depletion
Experiment
The experiment has been underway since the spring of 1996. Figures 3a and 3b shows the temporal trend in G-value in selected unheated test chambers for 239Pu and 238 Pu, respectively, with several simulated waste materials at approximately bi-weekly intervals. Also shown, for comparison, are G-values assigned to these materials by the TRUPACT II SARP. Results to date indicate that, in general, the rate of hydrogen generation decreases with time, as expected. In all cases, the measured G-values are well below those assumed in the TRUPACT II SARP. During 1997, after the hydrogen concentrations stabilize, we will introduce air back into the containers to observe the effect on hydrogen generation. We will also be exposing selected test chambers to agitation via vibration to investigate whether road vibrations that can be expected during transportation are sufficient to re-distribute the Pu particles on the surfaces of test matrix and result in an increased rate of hydrogen production.
The data from this experiment will be incorporated into an empirical model of gas generation/matrix depletion. This model will be the basis for assessing the hydrogen generation rate TRU waste drums as a function of the dose and contents of the drums.
Our expectation is that these experiments will permit an increase in the allowable thermal power limit by a factor of about 3 to 10. Referring to Fig. 1, this result would be sufficient to allow shipment of all of the drums in the 1<T<3 category (~3000 drums) and many of the drums in the 3<T<12 category (an additional ~3000 drums). In order to ship an even greater fraction of drums with high Curie loadings of Pu we are investigating measures that will remove hydrogen gas from the waste containers.
Hydrogen Getter Project
We are initiating an investigation into the use of hydrogen getters to continuously remove hydrogen from the headspace of waste drums and/or the TRUPACT-II.
Hydrogen recombiners and getters are two technologies that can remove hydrogen from the headspace gases of either the waste drums or the TRUPACT-II. While both technologies are effective at removing hydrogen, the getters offer attractive advantages in fabrication flexibility and performance. Hydrogen recombiners are noble metal catalyst beds that remove hydrogen gas by reaction with oxygen to form water. However, the NRC has rejected the use of recombiners for removing hydrogen from the TRUPACT II because of:
Hydrogen getters are solid materials that scavenge hydrogen from the gas phase and chemically and irreversibly binds it in the solid state. Hydrogen getters do not require the presence of oxygen to be effective. They do not produce water as a reaction by-product.
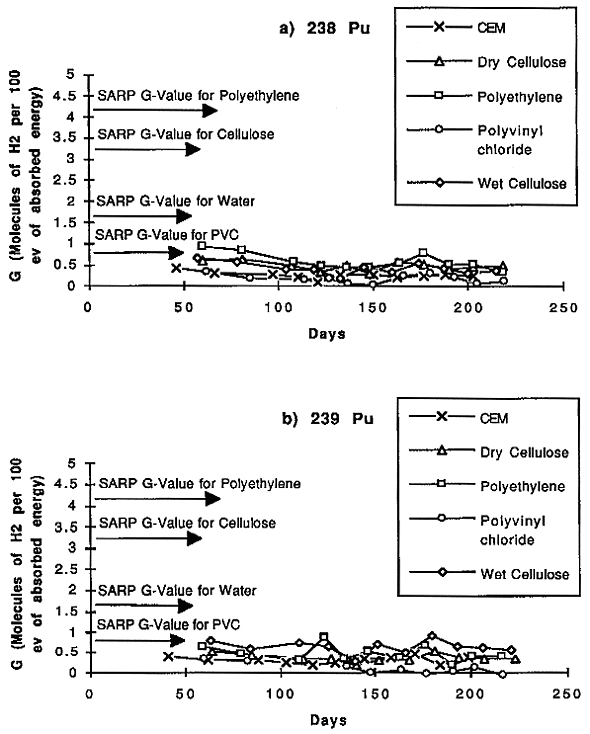
Fig. 3. Hydrogen concentration trends
in chambers containing simulated waste materials and a) 238Pu and b)
239Pu.
Hydrogen getters belong to a class of organic compounds called alkynes that are characterized by the presence of carbon-carbon triple bonds. In the presence of suitable catalysts such as palladium (Pd), hydrogen will add across the unsaturated bonds to form the corresponding saturated alkane.
Compounds of this class were originally developed for use in nuclear weapons by Sandia National Laboratory and the Kansas City Division of Allied-Signal. They are used to protect electronic components in sealed assemblies from hydrogen corrosion. They are also currently being used to provide secondary confinement for tritium shipping containers.
Many compounds and formulations have been tested (Smith, 1992; Smith et al., 1990). The best performance has been achieved with 1,4-bis(phenylethynyl)benzene (DEB) (Fig. 4). It has a capacity of 240-330 cm3 (STP) hydrogen per gram DEB. DEB is a non- toxic, non-mutagenic, crystalline solid. Because it is a di-alkyne (containing two triple bonds), one mole of DEB reacts with 4 moles of hydrogen. It melts at 179°C; whereas the fully hydrogenated product melts at 87°C. The standard formulation for the DEB getter is a mixture of 75% DEB and 25% carbon catalyst (5% Pd on carbon). The production process is quite simple: the two materials are mixed together in a ceramic jar mill for several hours, after which the DEB getter is ready for use. It has been shown to be stable in the absence of hydrogen for up to 18 months (at 70°C, under N2). The DEB formulation has been successfully incorporated into several forms including powder, pellets, shaped polyethylene composite, a urethane adhesive film and a castable RTV silicone. The material has been in regular production for use in DOE components and assemblies since 1977.
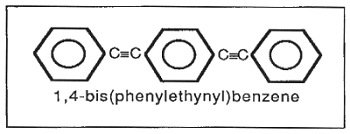
Fig. 4 Structure of
1,4-bis(phenylethynyl)benzene (DEB)
The DEB getter reacts rapidly, exothermically and irreversibly with hydrogen. The reaction is nearly stoichiometric and proceeds to >90% of the theoretical capacity. In experiments in a nitrogen atmosphere with a hydrogen addition rate of about 10-3 cm3/sec, the hydrogen concentration was maintained at less than 5 ppm until the getter had reacted with >90% of its theoretical capacity. The reaction rate with hydrogen is temperature dependent and proceeds more rapidly as the temperature is increased.
Little testing has been done on the effects of other gases on the DEB getter. Performance is reportedly unaffected by methylene chloride, ammonia or by carbon monoxide at concentrations of less than 1% (H. M. Smith, personal communication, 1996). Hydrogen sulfide is a well-known poison of noble metal catalysts and, because of the presence of Pd-carbon catalyst in the DEB getter formulation, it may inhibit the performance of the DEB getter as well. However, hydrogen sulfide is not commonly encountered in TRU waste headspace gases. Nevertheless, the performance of DEB in the presence of volatile organic compounds (VOCs) known to be present in TRU waste headspace gases is of concern and is the primary focus of our research.
It is not necessary to test DEB in the presence of every VOC that could possibly be present in TRU waste headspace gases. Rather, we are examining databases of VOC analyses of drum headspace gases and grouping the compounds into targeted chemical families with common functional groups: e.g. alcohols, ketones, chlorinated hydrocarbons, etc.
The experimental approach will be to pass a mixture of hydrogen and the target chemical family being tested in air through a column of DEB. A control column will be exposed to a gas stream with the same concentration of hydrogen but not containing the potential poisons. Comparison of the time of appearance of hydrogen from the test column and the control column will be a measure of the toxicity of the target poisons. If hydrogen appears from both columns simultaneously, then the target compounds are not poisonous to DEB. If hydrogen first appears from the test column then, the DEB is being poisoned by the target gases and diminishing its performance with respect to hydrogen.
If we discover compounds that significantly poison the DEB getter, we will investigate encapsulation of DEB within selective polymeric and/or metal membrane materials (Peachey et al., 1995, Peachey et al., 1996) that allow hydrogen to pass but will exclude any other gases including those that may poison the DEB getter.
CONCLUSION
The results of the Matrix Depletion Project and Hydrogen Getter Project will form the technical justification for petitioning the NRC for to increase in the TRUPACT-II wattage limits. This will permit more waste to be shipped to WIPP without requiring expensive repackaging, treatment, or the development of alternative shipping packages.
BIBLIOGRAPHY
ZERWEKH, A. Gas generation from radiolytic attack of TRU-contaminated hydrogenous waste. LA-7674-MS, Los Alamos National Laboratory, Los Alamos, NM, 1979.
KOSIEWICZ, S. T., Gas generation from organic transuranic wastes. I. Alpha radiolysis at atmospheric pressure, Nuclear Technology, 54, 92-99, 1981.
ZERWEKH, A. and J. L. WARREN, Gas generation and migration studies involving recently generated Pu-238-contaminated waste for the TRU waste sampling program., LA-10732-MS, Los Alamos National Laboratory, Los Alamos, NM, 1986.
Marshall et al., Determining Site-specific drum loading criteria for storing combustible 238Pu waste. LA-US-94-409, Los Alamos National Laboratory, Los Alamos, NM, 1994.
SMITH, H. M., T. J. SHEPODD, L. R. GILLIOM, Organic getter materials for the removal of hydrogen and its isotopes, Minerals, Metals and Material Society Fourth International Conference on Hydrogen Effects on Material Behavior, 1990.
SMITH, H.M. 1992. Facsimile communication to J. Krsul, Argonne National Laboratories-West. October 23, 1992.
US Patent filed June 7, 1995 "Composite metal membrane" (N. M. Peachey, R. C. Dye, R. C. Snow, S. A. Birdsell).
N. M. PEACHEY, R. C. SNOW, R. C. DYE, J. Membrane Sci., in press, 1996.