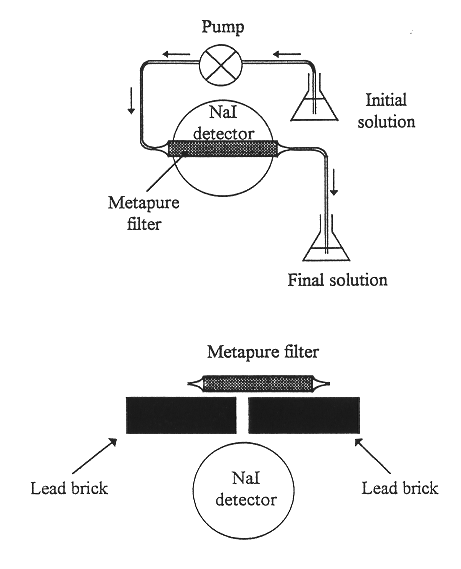
Fig. 1. Experimental set up for
uptake and distribution measurement of the radioactive tracer in the Metapure
filter.
E. Tel Or and N. Cohen
The Hebrew University of
Jerusalem
Faculty of Agriculture, Rehovot, ISRAEL
Tel. 972 - 8 -
9481262, Fax 972 - 8 -9467763
D. Ilzycer, I. Gilath, A. Mei-Marom and H. Zafrir
SOREQ
Nuclear Research Center, Yavne 81800, ISRAEL
Tel. 972 - 8 - 9434134, Fax
972 - 8 -9434403
ABSTRACT
A new biomass, Metapure, based on a dried plant is presented. It binds and concentrates radioactive and heavy metal ions from water solutions in a broad range of concentration (ppm to below ppt) and over a wide range of pH: 2-11. Metapure biomass is suitable for clean-up of nuclear industry waste water from radioactive and toxic metal ions. The biomass can be incinerated at temperature of around 500°C, to heavy metal enriched ashes, reducing drastically the weight of the waste for disposal up to 1/10.
Few examples of radionuclides accumulation by the biomass, from various solutions representing clean up problems, are presented.
INTRODUCTION
Metapure is a patented material based on a dried plant (biomass). It has remarkable properties of selective binding and concentration of radioactive and heavy metal ions removal from waste water in the ppm to below ppt concentrations and over a wide range of pH: 2-11. In real tests, Metapure was found to bind and concentrate metal ions in solution at ultra low concentrations, much below ppt.
The binding capacity for heavy metals is high, for example Metapure can bind lead (Pb) in a quantity equivalent to 10% of its dry weight. Metapure is a green product and does not release toxic components in the treated waste water. It is easily incinerated at low temperature (~ 300 - 500°C) to heavy metal enriched ashes, reducing the weight of the biomass waste for disposal up to 1/10. Metapure in the dry form is chemically stable and has an extensive shelf life.
Filtering system utilizing Metapure biomass is suitable for remediation of nuclear industry waste water from radioactive and toxic metal ions.
METHODOLOGY
Solutions containing metal ions with radioactive tracers such as Zr, Hf, Cs, Ru, Ce and Co were tested in the laboratory. The metal ions concentrations were in the ppm, ppb and ppt ranges. Similar experiments were performed with non-radioactive metalions such as Hg, Pb, Cd and others. The removal of most of the above ions by the biomass, was close to 100%.
The experimental laboratory set-up consisted mainly of a vessel for the feed solution, a peristaltic pump to flow the feed solution through a packed column with Metapure, a vessel for the effluent and a NaI nuclear detector for activity measurements (Fig.1).

Fig. 1. Experimental set up for
uptake and distribution measurement of the radioactive tracer in the Metapure
filter.
The laboratory column was 1.5 cm diameter and 15 cm length and contained 5 g of dry biomass. The flow rate experimented were from 2-120 ml/min. During the flow experiments through the biomass, the column was placed in front of the NaI detector for continuous real time measurement of radionuclides uptake in the column. The metal ion concentration was determined by activity measurements on feed and effluent solutions as well as the metal ion uptake on the Metapure column, allowing calculation of material balance.
The distribution of the metal ions on the Metapure column was determined by shielding the column with lead bricks leaving a slit toward the detector. The column was then slided along the slit and the activity recorded as a function of the column length.
The concentration of non-radioactive heavy metal ions was measured using ICP techniques. The metal ions concentrations in solution were measured directly. ICP analysis of metal ion accumulation on Metapure were performed after acid digestion of the biomass or ashing prior to acid digestion.
RESULTS
In order to demonstrate the efficiency of the biomass, results are presented for few examples of experiments.
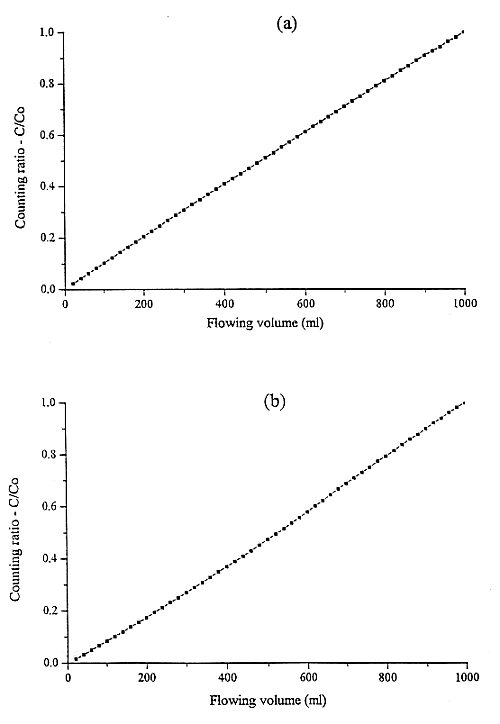
Fig. 2. Biomass uptake for Cs-134 at
100 ppb (a) and 5 ppm (b) solution concentration.
Table I Metapure and Dow HCR-SLE resin uptake of radionuclides from
IRR1 reactor pool cooling water.
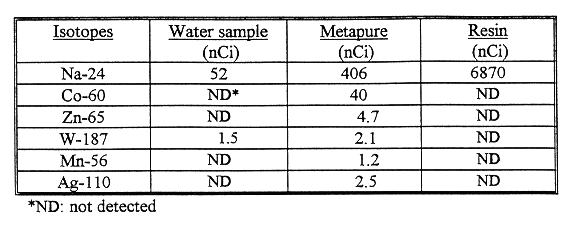
Table II Composition of Simulant Solution of Radioactive Waste
Water
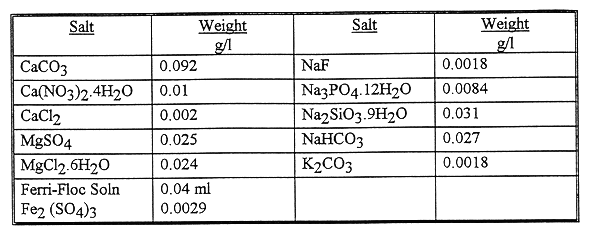
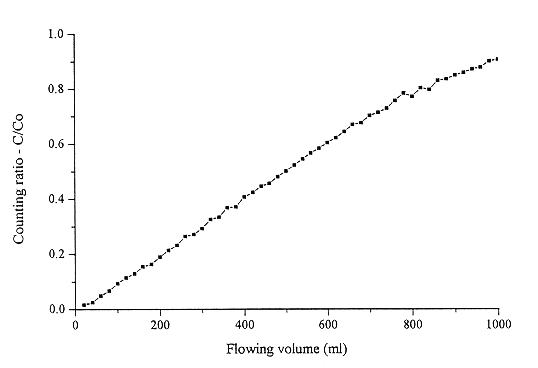
Fig. 3. Cs-134 uptake from Oak Ridge
simulant waste water solution.
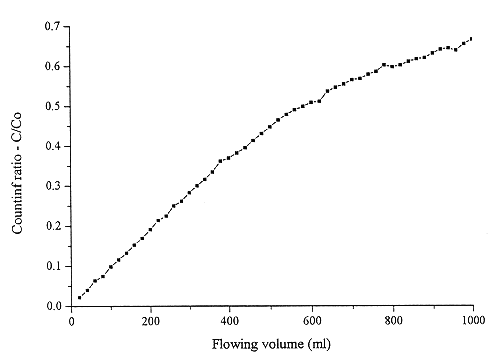
Fig. 4. 2 ppb Cs-134 uptake from high
salinity NaCl solution (20 g/l).
CONCLUSIONS
The efficiency of Metapure to remove trace quantities of radioactive or heavy metal ions was shown. The Metapure biomass has selective removal properties. Metapure biomass can be used efficiently for the clean up of nuclear industry waste water from radioactive and toxic metal ions.
REFERENCE