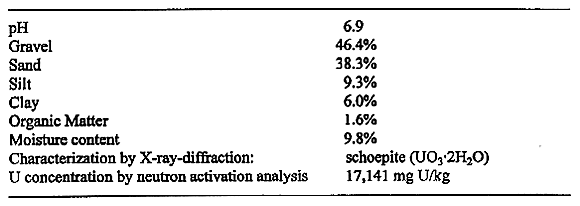
Table I. Characterization of representative contaminated soils.
W. R. J. R. Turney, N. P. Lu, C. F. V. Mason, M. C. Duff, and
D. E. Dry
Los Alamos National Laboratory
ABSTRACT
An environmental pilot treatment study for the remediation of uranium-contaminated soil by use of a two-step, zero-discharge, 100% recycle system was conducted at a former weapons research site. In the first step, following excavation, the soil was sorted by use of the ThemoNuclean Services segmented gate system (SGS) into two different streams: One stream with radioactivity greater than 30 pCi/g, the other steam with radioactivity less than 30 pCi/g . Following the sorting, the soil with radioactivities less than 30 pCi/g was returned to the excavation site as it met regulatory soil release guidelines. The portion of soil with radioactivities greater than 30 pCi/g was chemically treated in a second step containerized vat leach process by use of sodium-bicarbonate leach solution. The results of the second step are reported here.
OBJECTIVE
The two objectives of the pilot treatment study were: 1) to chemically leach uranium from the SGS segregated soil, by use of the containerized vat leach (CVL) system so that the activity concentrations of soil produced a total dose of less than 15 mrem/yr. (as an approximation the total activity from uranium must be less than 114 pCi/g) and thereby permitting the soil to be returned to the excavation site, and 2) to remove uranium from the leach solution by use of ion exchange resin. (Soil sorting criteria for the SGS was to divert the soil with greater than 30 pCi/g to the CVL system for treatment.) Following treatment the resins were to be either disposed of in an approved low-level radioactive landfill, or would be recycled by removal of the uranium from the resins.
INTRODUCTION
The site selected for this pilot study is situated at Technical Area 33 (TA-33) near Building 16 (TA-33-16) at an area known as Area 6, developed in 1948 for initiator experiments. TA-33-16 housed a military air gun for the firing of uranium projectiles as part of nuclear weapons research. Another gun was stationed and fired from a concrete pad adjacent to TA-33-16. The projectiles were fired at targets that were placed in front of two catch boxes and located less than 50 feet south of TA-33-16. Experiments at Area 6 were discontinued in 1955.
The two catch boxes, constructed of timbers, were 1.5 m wide, 3.0 m deep, and 3 m in height and were located less than 15 m south of TA-33-16. The boxes were filled with soil, wood chips, and vermiculite. The targets were constructed, in part, of uranium, beryllium, and polonium-210 (alpha emitter, t½ = 138 days). The projectiles often cracked open, contaminating the concrete and asphalt pads adjacent to TA-33-16, and the surrounding area, with the uranium, beryllium, and polonium-210. Initially the projectiles were constructed of tuballoy (natural) uranium. About ten percent of the projectiles were alloyed with one to two percent molybdenum to increase their compressive strength. Occasionally boron carbide was used as it is an effective neutron reflector. Titanium was alloyed either with the uranium or with the outer steel casings to decrease the weight in some instances (1). In 1994 the timbers of the catch boxes were disintegrating but still in place.
An environmental investigation, conducted in 1994 at the direction of the Los Alamos National Laboratory (LANL) Environmental Restoration (ER) program, determined the existence of uranium-contaminated soil within the catch boxes (2). The western catch-box contained oxidized uranium in a "pocket." Radioactivity in this contaminated zone, measured in the field, was as high as 13,000 cpm using a direct reading beta/gamma instrument. Another larger "pocket" of oxidized uranium was located between the two catch boxes. Radioactivity in this slightly larger contaminated zone was as high as 135,000 cpm beta/gamma.
Two soil samples from the boxes contained elevated uranium concentrations (216 mg/kg and 6,770 mg/kg). The remaining samples had uranium concentrations ranging between 2 mg/kg to 6 mg/kg uranium (indicating background concentrations of uranium in the soil (3)).
The excavation work in these catch-boxes indicated the uranium contamination was heterogeneously distributed. Due to extent of uranium found within the now former catch-boxes, the trenches were backfilled in 1994 and tarps were placed over the soil. The catch boxes were covered with high density polyethylene (HDPE) in 1995 as part of an Interim Action to prevent erosion of soil.
Preliminary environmental remediation sampling revealed the presence of uranium in the catch boxes. However, no beryllium, lead, or high explosives were detected in the catch boxes. Thus it was selected for a pilot-scale study of chemical leach removal of uranium.
CLEANUP REQUIREMENTS
Preliminary numerical modeling calculations were completed to determine the allowable concentration of uranium in the soil to meet regulatory requirements of concern. 10 CFR 834.305 states authorized limits for radionuclides in soil shall be derived using approved models for deriving soil criteria and ALARA (4). RESRAD (a Department of Energy (DOE) approved computer model "Residual Radioactive Material") was developed by the Argonne National Laboratory for the DOE for deriving soil criteria when conducting decontamination efforts. RESRAD was run to meet the following requirements:
The calculations assumed recreational scenarios for a trail hiker and picnicker exposed for typical recreational time periods. The area of contamination was assumed to be 1,500 square meters and thickness of area was set at 3 meters. The highest concentration of uranium found in the catch boxes (6,770 mg/kg, 4,800 pCi/g) was used as the initial soil uranium concentration.
Results from the calculations indicate that a person hiking will receive an exposure of 198 mrem/yr., or picnicking by the pile (with the above dimensions) will result in an exposure of 639 mrem/yr. In order for the trail hiker not to exceed a dose limit of 15 mrem/yr., the uranium concentration of the soil must be reduced by a factor of 13 (to 369 pCi/g). In order for the picnicker not to exceed a dose limit of 15 mrem/yr., the uranium concentration of the soil must be reduced by a factor of 43 (to 114 pCi/g).
In order to meet the most conservative dose limit and scenario, the combined ThermoNuclean and containerized vat leach procedures must be capable of detecting and removing uranium contamination to as low as 114 pCi/g.
SOIL TREATMENT METHODS
The contents of the catch boxes were excavated and a total of 353 metric tons (~156 m3) of uranium-contaminated soil was processed by use of the ThemoNuclean Services segmented gate system (SGS). (ThermoNuclean is a subsidiary of ThermoNutech, formerly ThermoAnalytical/Eberline). Of the 353 metric tons of soil sorted, 8.9 metric tons were diverted as being above 30 pCi/g ; this resulted in an initial waste volume reduction of 97.5%. The containerized vat leach system (CVL) system was used to leach uranium from the 8.9 metric tons of diverted soil.
Containerized Vat Leach System
The CVL process involves leaching uranium contaminated soils with 0.5 M NaHCO3 (sodium-bicarbonate) (pH~8.3) to selectively dissolve the uranium.
Carbonate (CO32- ) and bicarbonate (HCO3-) leach has historically performed well to recover uranium (U) from ore (5). More than one-third of the uranium mills operated in the United States have employed carbonate leaching circuits at one time or another. The use of carbonate heap leach requires the use of an integrated and closed circuit process, wherein the leach solutions are recycled and the reagents are reused.
Carbonate salt leach solution has two important roles. The formation of highly soluble anionic carbonate uranyl species, including uranyl dicarbonate (UO2(CO3)22-) and uranyl tricarbonate (UO2(CO3)34-), allows for high concentrations of uranium in a leachate solution. Secondly, carbonate salts are nearly selective for dissolution of uranium from uranium contaminated soils. Compounds of iron, aluminum, titanium, etc., are nearly insoluble in carbonate solution and are largely separated from the uranium during leaching. Oxidized uranium minerals are readily soluble in carbonate solutions. Other advantages of the carbonate leaching process include high solubility, the purity of the solution produced, the relative ease with which a uranium product can be precipitated directly from the leachate solution, and the relatively noncorrosive and safe handling characteristics of carbonate solutions.
From the chemistry of uranium, it is known that oxidized hexavalent uranium (U6+) in the form of uranyl ion (UO22+) will be easily solubilized to a carbonate complex whereas reduced tetravalent uranium (U4+) will be unaffected by the presence of carbonate. The stages of solubilization have been documented by Grenthe (6):
UO22+ + CO32- = UO2(CO3)0 (1)
UO22+ + 2HCO3- = UO2(CO3)22- + 2H+ (2)
UO2(CO3)22- + CO32- = UO2(CO3)34- (3)
UO2(CO3)22- + HCO3- = UO2(CO3)34-+ H+ (4)
The overall reaction for the dissolution of uranium oxide in bicarbonate solution to uranyl tricarbonate is as follows:
UO22+ + 3HCO3- = UO2(CO3)32- + 3H+ (5)
The CVL process involves application of the bicarbonate solution to uranium-contaminated soil. This dissolves (leaches) uranium in the form of uranyl carbonate ion. The amount of uranium that is leached is primarily a function of the characteristics of the soil and the uranium speciation.
The bicarbonate solution is continually recycled through the soil and the soluble uranium removed onto ion exchange resins.
The soil was leached in commercially available inexpensive containers manufactured by Rinchem Company, Inc. (Rinchem constructed the bags pursuant to design specifications from LANL). The bags are reusable.
The Containerized Vat Leach containers had a volume capacity of 0.77 m3. The containers are constructed of a woven polyethylene material and have a 3.0 metric ton capacity. The containers have a plastic liner. An elephant trunk 150 mm. diameter and 150 mm in depth was sown into the bottom of the container allowing liquids introduced to the container to drain out the bottom center of the bag. The elephant trunk also acts as a filter.
The CVL containers, once filled with soil, were hung from steel racks. A leach solution reservoir was placed under the CVL container.
The soil was deposited directly into these bags after sorting by the segmented gate system. The soil was then treated with the leach solution. After passing through the bag, and a 10 µm elephant trunk filter at the bottom of the CVL, the leach solution was pumped from the reservoir through two filters, a 10 µm filter and a 1 µm filter, before being recycled back to the top of the bag. Latterly, the leach solution was also being passed through an ion exchange column to remove the uranium solubilized as a carbonate complex.
Analytical Methods
The following analytical methods were used for chemical characterization of the solutions and soils used in the pilot project: alkalinity, gamma spectroscopy, inductively coupled plasma - atomic emission spectra (ICP-AES), kinetic phosphoresence analysis (KPA), x-ray diffraction (XRD), scanning electron microscope (SEM), kinetic phosphorescence analysis, and pH.
Ion Exchange Experiments
Treatment of these liquors is important to reduce the volumes of the waste, and to recover and to recycle the bicarbonate reagent. Anion-exchange resins are effective absorbers for removing U from the bicarbonate leach liquors of TA-33 site soil. In site scaleup experiment, U was successfully removed uranium-bicarbonate leach liquors using an anion-exchange column system.
RESULTS
Table I shows the characterization of soil from a site at TA-33 that is similar to that studied in the pilot treatment study.
Table I. Characterization of representative contaminated soils.

Equilibrium geochemical modeling of leach solutions showed that given the concentration of uranium in the soil and the molarity of the leach solution, the solution would be 2 to 4 times undersaturated w.r.t. schoepite. The major uranium species would be UO2(CO3)22- and UO2(CO3)34-.
Uranium solids present in the soils were also analyzed by XRD and were identified as schoepite (UO3*2H2O).
A total of 383,109 kg of soil were processed by the SGS, and 1.66E9 pCi of activity were diverted in 9.7 metric tons of soil for chemical remediation by containerized vat leach. Nine containers, each containing approximately one ton of uranium-contaminated soil, were then processed.
Results for CVL_3 Container
The first containerized vat leach container processed was denoted as CVL_3 and was processed singularly (later bags were processed in tandem). Aqueous solution leach solution samples were taken at regular intervals and analyzed for pH and uranium (by KPA). These results are shown in Table II.
Table II. Leach results from CVL bag No. 3 (denoted CVL3).
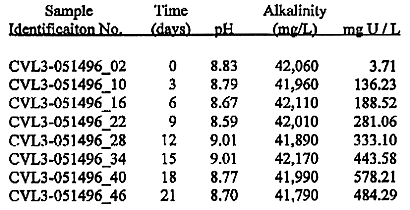
When the majority of the uranium had been removed, the soil itself was sampled for residual uranium. The results of the soil analysis post leach indicted uranium concentrations of ~46 p/Ci from 234U, ~2.02 pCi/g from 235U, and ~46 pCi/g from 238U, for a total uranium activity of 94 pCi/g.
Following the leach of CVL_3 solid pieces of schoepite 6 mm in diameter or larger were observed in the top 50 mm of the soil in the CVL. It was decided that future soils to be leached in the CVLs would be sieved first, sieving out the larger than 6 mm fraction. The remaining less than 6 mm fraction of the soil would be chemically leached in the CVLs. CVLs that were leached concurrently with CVL_3 were also sieved post leach, removing the larger than 6 mm fraction, and thus a large amount of the remaining radioactivity in the soil.
Results from all the CVL Containers
Besides CVL_3, two (CVL_1 and CVL_6) met the DOE criteria for release (114 pCi/g). Both of the containers were pre-sieved, sieving out pieces of shoepite that were larger than 6 mm in diameter, and were both leached for 15 days each. There was a 97 percent and 90 percent reduction, respectively, of uranium in the soils in the containers by the pre-sieve and leach process. CVL_4 nearly met the criteria (117 pCi/g) after 18 days of leach, resulting in a 67 percent reduction of uranium-contamination. CVL_4 was not pre-sieved. CVL_2 and CVL_5 were both above the release criteria (171 pCi/g and 123 pCi/g, respectively). Reduction of uranium contamination was 86 percent and 91 percent, respectively, in these two containers. CVL_2 was leached for 14 days, and was sieved for shoepite larger than 6 mm following the leach process. CVL_5 was leached for ten days and was sieved following leach. Percent uranium reduction in CVL_2 and CVL_5 was 86 percent and 91 percent, respectively. The other three CVL containers (CVL_7, CVL_8, and CVL_9) were all pre-sieved. Analysis of CVL_7, CVL_8, and CVL_9 are pending.
Filters and Sludge from CVL 3 Bag.
Ion Exchange for Uranium Removal from Leach Solutions
The initial sodium bicarbonate leach liquors collected from vat leach bag CVL_3 had pH 9.82 and contained uranium, silica (Si), magnesium (Mg), potassium (K), calcium (Ca), lead (Pb), aluminum (Al), and iron (Fe) (see Table III). During the leaching process, the leach solution was continuously passed through a filter system including one of 10 µm deionization system filter column and one 1 µm filter column. The concentrations of U, Ca, Mg, K and Si in the leach solution were reduced by filtration in the recycle. This filtered solution was used in the ion exchange studies including batch and column experiments.
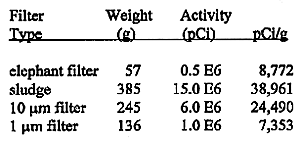
Table III shows the weight of solids collected on the elephant filter, the sludge at the bottom of the leach collector container, and the two filters - 10 µm and 1 µm.
Table III. Weight s and Activity Found on Filters and Sludge.
Plastic columns, capacity ~ 3 L, were used to construct a multi-column system. Four columns were installed in series. At the bottom of each column, a filter pare (20 µm mesh) and a mesh screen (<0.5 mm mesh) were placed on top of a porous plane to support the resin beads. About 2 L of anion-exchange resin, Ionac(TM) SR-3 (resin bead size of 200 µm to 500 µm) were placed into each column. The filtered uranium-bicarbonate leach liquors were introduced into the columns at a flow rate of 1.7 L/h.
Generally, after 210 liters of uranium-bicarbonate leach liquors were passed through four columns of Ionac(TM) SR-3 resin, approximately 95.3% of uranium in the leach liquors was removed by the resin. The site scaleup column test showed that the Ionac(TM) SR-3 is a effective sorbent to remove uranium from relative large amounts of the bicarbonate leach waste stream of the soil. Lu (7) describes in detail the results and analysis of the entire ion exchange experiments.
SUMMARY
Of the six CVL containers leached to completion to date, three met the DOE criteria for release (114 pCi/g). Both of those containers were pre-sieved, sieving out pieces of shoepite that were larger than 6 mm in diameter, and were both leached for 15 days each. There was a 97 percent and 90 percent reduction, respectively, of uranium in the soils in the containers by the pre-sieve and leach process. CVL_4 nearly met the criteria (117 pCi/g) after 18 days of leach, resulting in a 67 percent reduction of uranium-contamination. CVL_4 was not pre-sieved. CVL_2 and CVL_5 were both above the release criteria (171 pCi/g and 123 pCi/g, respectively). Reduction of uranium contamination was 86 percent and 91 percent, respectively, in these two containers. CVL_2 was leached for 14 days, and was sieved for shoepite larger than 6 mm following the leach process. CVL_5 was leached for ten days and was sieved following leach. Percent uranium reduction in CVL_2 and CVL_5 was 86 percent and 91 percent, respectively. The other three CVL containers (CVL_7, CVL_8, and CVL_9) were all pre-sieved and analysis are pending.
Ionac(TM) SR-3 ion exchange resin was used to treat the leach solution, resulting in 95.3 percent recovery of uranium from the leach solution onto the resin.
RECOMMENDATIONS
When working with soils containing solid pieces of uranium-oxides, it is recommended that the soils be pre-sieved, separating out the larger than 6 mm diameter fraction. This physical treatment will result in a stream of material of that will contain large radioactivity from particles of uranium-oxide larger than 6 mm. The smaller than 6 mm fraction, although greatly reduced in radioactivity but still greater than the release criteria, may then be treated chemically to reduce the uranium-contamination to less than the release criteria dictated by the RESRAD release criteria model.
ACKNOWLEDGMENTS
Thanks to Roy A. Michelotti of the Los Alamos National Laboratory Environmental Restoration Project for funding this project. Thanks also to Ines R. Triay, Andrew I. Adams, Charles R. Cotter, Dorothy Hoard, Wayne W. Lemons, S. Fredric Marsh, John A. Musgrave, Bradley S. Schake, and Don A. York.
REFERENCES
GLOSSARY
Al aluminum
ALARA As Low as Reasonably Achievable
Ca calcium
CFR code of federal regulations
CO32- carbonate ion
cpm counts per minute
CVL containerized vat leach
DOE Department of Energy
ER Environmental Restoration
Fe iron
g gram
HCO3- bicarbonate ion
HDPE high density polyethylene
ICP-AES inductively coupled plasma - atomic emission spectra
kg kilogram
K potassiumKPA kinetic phosphoresence analysisKPA kinetic phosphorescence analysis
LANL Los Alamos National Laboratory
m3 cubic meter
M molarity
mg milligram
mrem millirem
Mg magnesium
NaHCO3 sodium-bicarbonate
pCi pico-curie
pH negative log of the hydronium ion concentration
Pb lead
rem "rad-equivalent man"
RESRAD Residual Radioactive Material
SEM scanning electron microscope
SGS segmented gate system
Si silica
t½ half life
TA-33 Technical Area 33
U uranium
U4+ tetravalent uranium
U6+ hexavalent uranium
UO2(CO3)22- uranyl dicarbonate
UO2(CO3)34- uranyl tricarbonate
U(VI) hexavalent uranium
UO22+ uranyl ion
U(IV) tetravalent uranium
UO2(CO3)0 uranyl monocarbonate
UO3*2H2O schoepite
µm micrometer