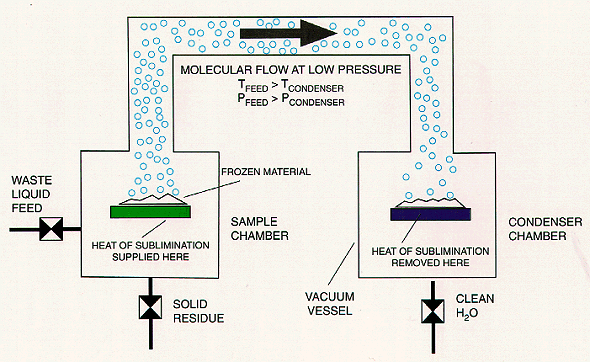
Fig. 1. Concept of low temperature
sublimination (freeze drying)
John A. Musgrave, D. Wes Efurd, Joseph C. Banar, Stephanie
Boone
Chemical Science and Technology Division
Los Alamos National
Laboratory
Los Alamos, NM 87545
Richard A. Podkulski, Ernesto Renzi, and Paul Stewart
BOC Edwards Calumatic, 2175 Military Rd.
Tonawanda, NY 14150
ABSTRACT
The Department of Energy must comply with ever increasingly stringent standards for the levels of radioactive and non-radioactive materials present in liquid effluents. Current conventional methods of decontamination include distillation, ion exchange, precipitation reactions, or chelating agents, require multiple passes, produces secondary wastes (resins, chelating agents), and have resulted in much lower separation factors. Freeze drying technology (FDT) has been applied to the separation of radioactive and non-radioactive liquids and shown to be thousands of times more effective than conventional methods in use. This technology addresses problems in the areas of waste minimization and pollution prevention.
Freeze drying is the removal of solvent or volatile components by sublimation from a frozen solution, suspension, or multicomponent material. The freeze drying process involves two independent steps: freezing of the material and removal by sublimation (drying) of the volatile component. FDT will efficiently separate solvents and volatile acids from complex waste solutions and process liquids without generating secondary wastes. The separated liquids will be virtually free of radioactive contamination and/or RCRA metals and can be re-used or discarded as non-mixed waste or even non-radioactive. FDT will thus eliminate the need for storage or destruction of the liquid component and will lower storage and transportation costs because of volume reduction and weight reductions. FDT can be considered safe; no high temperatures or pressures are used. The process occurs in a vacuum, so failure of a component would lead to an inward leak and the potential for contamination outside the system is significantly reduced. The refrigerant is environmentally friendly liquid nitrogen; no CFCs are used. Hence, FDT has the potential of reducing the volume of radioactive, mixed, and hazardous wastes in an environmentally responsible manner.
INTRODUCTION AND BACKGROUND
The Department of Energy (DOE) must comply with ever increasingly stringent standards for the levels of radioactive and non-radioactive materials present in liquid effluents. In addition, industries such as metals, chemical, electronic, and petroleum refining too must comply with increasingly stringent standards for the levels of hazardous materials present in liquid effluents. Current conventional methods for separating undesirable substances from waste streams include distillation, ion exchange, precipitation reactions, or chelating agents, require multiple passes, produces secondary waste (resins, chelating agent), and have resulted in much lower separation factors. Freeze drying technology (FDT) has been applied to the separation of heavy metals in liquids and shown to be thousands of times more effective than conventional methods in use. FDT will efficiently separate solvents and volatile acids from complex waste solutions and process liquids. The separated liquids will be virtually free of radioactive and hazardous components and/or RCRA (Resource Conservation and Recovery Act) metals and can be re-used or discarded as non radioactive and/or non-hazardous waste. This technology addresses problems in the areas of pollution prevention and waste minimization.
Freeze drying is the removal of solvent or volatile components by sublimation from a frozen solution, suspension, or multicomponent material. The freeze drying process involves two independent steps: freezing of the material and removal by sublimation (drying) of the volatile component. The removal of a solvent under these conditions often results in the preservation of the solid portion of the material. This preservation aspect of FDT has led to diverse applications that have resulted in the processing of tons of material daily throughout the world. The objective of this work is to demonstrate the feasibility of FDT to the separation of complex radioactive and non-radioactive materials including liquids, slurries, and sludges containing a wide variety of constituents in which the separation factors are in excess of 108. When FDT is applied to the separation of multicomponent radioactive materials, the product is the condensate, as opposed to the solid portion of the material. This is the first application of FDT where primary importance is given to the condensate. Our focus is applying this technology to the elimination of radioactive discharges from facilities at Los Alamos and within the DOE complex; however, successful demonstration will lead to nuclear industry-wide applications.
An aqueous solution can be separated into the solvent and solute components in the following manner (Fig. 1). The solution is frozen; the frozen material is situated in the proximity of a condensing surface which is colder than the frozen solution (T1 > T2). A concentration gradient is established between the frozen radioactive solution in the sample chamber at left and the condenser surface due to the difference in the temperature dependent vapor pressure (P1 > P2) of the solvent at the two different locations. This concentration gradient drives the transport of the nonradioactive solvent from the sample chamber to the condenser. A vacuum is usually applied to facilitate the diffusion of the sublimed solvent since the vapor pressures above the frozen solution can be quite low. Complete solvent removal is achieved by supplying enough heat to the frozen material to drive the sublimation process while removing enough heat at the condenser to maintain the concentration gradient (1).

Fig. 1. Concept of low temperature
sublimination (freeze drying)
Nuclear waste streams have relatively low solute concentration in comparison to, for example, foods. Consequently, the greatest impediment to the freeze drying rate, for nuclear waste solutions, is the thermal conductivity of ice. This fundamental limitation is circumvented through engineering solutions, such as maximizing surface of the freeze plates, minimizing the thickness of the frozen material, and supplying radiant energy directly to the surface of the frozen material.
Freeze dryers are extremely versatile and broad in their applications. Commercially available laboratory dryers are capable of subliming kilograms of solvent per day, whereas industrial dryers are capable of drying hundreds of kilograms per day or more. The most common industrial application of freeze dryers is in the pharmaceutical and food industries. The factor that most greatly influences the cost of a freeze dryer is the performance required by the particular application.
Application of FDT to Nuclear Wastes
FDT has been applied to the decontamination of waste streams containing nuclear and hazardous materials with great success. Preliminary results of work at Los Alamos National Laboratory indicate separation factors of 6 x 105. The separation factor is equal to the ratio of the initial to final concentrations. Three early applications of FDT to waste streams include 1) acidic solutions containing U, Fe, Cr, and Ni originating from nuclear power plant operations have been decontaminated such that the concentration of radioactive materials was 1 pCi/L (four times lower than current Environmental Protection Agency emission levels) while the separation factor was 6 x 107 (2), 2) waste solutions containing Na and K nitrates, 137Cs, 90Sr, 91Y, 144Ce, 106Ru, and 131I were reduced to dry residues while the condensate was decontaminated by 1 x 107 (3), and 3) other radioactive elements, such as I (4), Pu (5), and Ru (6) have been concentrated in the freeze dried solid residues to produce radiochemically pure water.
FDT is versatile in that its application is not limited to aqueous solutions. Organic solvents can be separated from other liquids or solids. Liquids processed in this way may be reused or discarded as non-radioactive.
Other decontamination methods such as ion exchange, precipitation, or chelation have high ion specificity but require multiple stages, regeneration, neutralization, and/or filtration. They involve the addition of other components (resins, chelating agents, precipitating agents, etc.) that also require remediation. Integration of FDT into these processing lines may greatly increase process efficiency and decontamination effectiveness.
Distillation and/or evaporation separation processes involve continuous, often turbulent flow; thus, entrainment of the radioactive component is an unavoidable consequence. Separation factors of 104 are theoretically achievable for distillation, but values of about one to three hundred are typical in practice. The freeze drying process differs from distillation in that the transport process during freeze drying involves molecular flow and thermal energy of the radioactive material is much lower. These features are responsible for the virtual elimination of solute entrainment; consequently, separation factors obtained using FDT are extremely high.
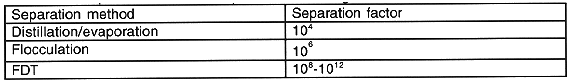
FDT drastically reduces the volume of the waste since the volume of solute or solid component is smaller than the solution volume. Volume reduction factors greater than one thousand have been achieved in aqueous nitrate/nitric acid solutions containing dissolved and suspended solids, but the exact volume reduction of waste depends on the particular moisture content. Volumes may also be reduced through elimination of neutralization steps in transuranic (TRU) processing. Processing of Pu- and U-contaminated scrap often involves the dissolution of the scrap in nitric acid. These solutions are usually neutralized using sodium hydroxide for compatibility with subsequent process steps. A significant percentage of the waste generated and consequently stored at Hanford was the result of this chemical neutralization. If a process similar to those conducted at Hanford were carried out today, the application of FDT may eliminate the use of neutralizing agents and consequently greatly reduce the quantity of waste solids at later process steps. Integration of FDT into process lines may greatly increase process efficiency, and when combined with other more conventional methods of waste stream separations, FDT can potentially increase the effectiveness of these separations up to 1012 (Table I).
Table I Comparison of Various Separation Technologies
FDT DEMONSTRATION
The FDT demonstration consists of two phases:
- Design, construct, and test a pilot-scale freeze dryer capable of processing up to 5 liters/day.
- Obtain design parameters for plant-scale freeze dryer.
The manufacturing and engineering capabilities required for the this project are beyond those available at Los Alamos. To meet this manufacturing and engineering need, Los Alamos has entered into a Cooperative Research and Development Agreement (CRADA) with BOC Edwards Calumatic, Tonawanda, NY, a commercial manufacturer of freeze dryers with capacities greater than 1000 liters/day/unit. BOC Edwards Calumatic is the industry leader in freeze drying equipment.
In this partnership, both parties have the opportunity for substantial gain. BOC Edwards Calumatic considers LANLs knowledge and experience with nuclear materials to be vital for the development of this new FDT application. They see this partnership as the foundation for development of a new product line and possibly new worldwide markets. Successful demonstration of FDT is crucial to meeting the immediate and long-range discharge goals from LANL facilities. Furthermore, particular nuclear waste problems facing other DOE sites such as Rocky Flats, Savannah River, and Hanford are also being considered in the demonstration of this technology. The DOE will gain indispensable technology for the treatment of legacy and newly generated wastes, and the nuclear industry in general will have at its disposal the technology to meet discharge limits that are becoming increasingly more stringent.
During the first phase of the project, we initially intend to conduct experiments with surrogate materials (e.g. sodium nitrate, various rare earth element salts) in aqueous solution. These surrogates with be stored in a 55 gallon drum. A Teflon-coated vane pump will transfer the solution to the sample chamber of the freeze dryer. The solution will be circulated or otherwise kept in motion during the freezing process. Once a 1 cm thick deposit of ice has accumulated on the freeze plates, the solution will be drained from the sample chamber and the sublimation process can begin. The solvent (in this case water and nitric acid) will be sublimed from the ice by a difference in temperature and pressure between the sample chamber and the condenser chamber. The solvent will freeze onto the coils in the condenser chamber. After the sublimation process is complete, the freeze dried material in the sample chamber will be collected in a tailings bucket at the bottom of the sample chamber. The frozen solvent in the condenser chamber will be allowed to melt, and this liquid will be collected and analyzed for the surrogate materials. From the concentration of solutes in the starting solution and the condensate, a separation or decontamination factor can be determined for that particular experiment.
These early experiments will be proof of principle. If these experiments are successful, we will conduct experiments with DU (depleted uranium), and if the experiments with DU are a success, we will conduct experiments with Pu.
If the proof of principle experiments are successful, then experiments designed to fully test the pilot-scale unit will be carried out. These experiments will constitute the second phase of the project. It is from these experiments that the design parameters necessary for the plant-scale freeze dryer design will be obtained.
COST, HEALTH AND ENVIRONMENTAL BENEFITS
Benefits of this technology to human health and the environment are several. FDT will eliminate the need for storage which carries the environmental impact of land utilized as the storage site and the risk of leakage from storage containers. There is also the cost consideration of storage. FDT will eliminate the need for destruction of the liquid component, which in the case of organic solvents may have air pollution restrictions. FDT will result in lower transportation costs because of the reduced volume and weight. Furthermore, FDT will save costs in the industrial process because water and materials such as acids can be recycled back into the process. This technology can be considered safe; no high temperatures or pressures are used. The process occurs in a vacuum, so the failure of a component would lead to an inward leak and the potential for contamination outside the system is significantly reduced. The refrigerant is environmentally friendly liquid nitrogen; no CFCs are used. Hence, FDT has the potential of reducing the volume of hazardous wastes in an environmentally responsible manner.
SUMMARY
FDT is a technology that can meet todays and tomorrows regulatory discharge limits for liquid effluents. The technology can process radioactive and hazardous waste from the DOE complex, the nuclear power, metals processing and plating, electronics, and chemical industries. Secondary wastes are not generated. Waste treatment using FDT can be done in a safe, environmentally responsible, and cost effective manner.
ACKNOWLEDGMENTS
The Los Alamos part of this project is funded by the U.S. Department of Energy Defense Programs through the Nuclear Materials and Stockpile Management Program Office at LANL. We would like to thank Randy Tremper and Donna Smith of the Industrial Partnership Office at LANL for managing various aspects of the CRADA and Dennis Southerland, BOC Edwards Calumatic, for his diligent work during the final stages of the proto-type design.
REFERENCES