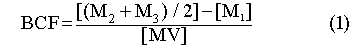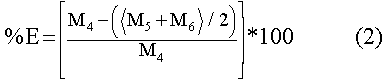INFLUENCE OF WATER HARDNESS ON ACCUMULATION AND
ELIMINATION OF CADMIUM IN TWO AQUATIC MOSSES UNDER LABORATORY CONDITIONS
C. Gagnon, G. Vaillancourt, L. Pazdernik
Laboratoire de
Recherche sur les Communautés Aquatiques, Université du Québec
à Trois-Rivières
Département de Chimie-Biologie, C.P.
500, Trois-Rivières,
Québec, G9A 5H7, Canada
ABSTRACT
This study investigated the effect of water hardness on the accumulation and
elimination pattern of Cd by two aquatic mosses, Fontinalis dalecarlica
and Platyhypnidium riparioides, under laboratory conditions. The two
mosses were exposed to nominal Cd concentrations of 0, 0.8, 2 and 10 µg•L-1,
which are comparable to concentrations found in nature. The influence of three
water hardness (very soft  15 mg•L-1,
soft
15 mg•L-1,
soft  45 mg•L-1 and hard
water
45 mg•L-1 and hard
water 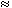 90 mg•L-1 as CaCO3)
was measured while maintaining the alkalinity and pH constant during the provide
duration here. The Cd accumulation by the aquatic mosses was rapid, showing the
potential of accumulation and sensitivity of this biomonitor. Even when the
actual Cd concentration in the water was low (concentrations <0.15 to 6.82 µg•L-1,
the uptake of Cd was very fast and mostly linear. Accumulation rates of Cd were
significantly different when the mosses were in very soft (
90 mg•L-1 as CaCO3)
was measured while maintaining the alkalinity and pH constant during the provide
duration here. The Cd accumulation by the aquatic mosses was rapid, showing the
potential of accumulation and sensitivity of this biomonitor. Even when the
actual Cd concentration in the water was low (concentrations <0.15 to 6.82 µg•L-1,
the uptake of Cd was very fast and mostly linear. Accumulation rates of Cd were
significantly different when the mosses were in very soft (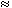 15 mg•L-1) as compared to hard
water (
15 mg•L-1) as compared to hard
water (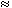 90 mg•L-1 as CaCO3).
The elimination of Cd followed a very slow process for the two species studied.
The elimination rates of Cd from the mosses were not influenced by water
hardness.
90 mg•L-1 as CaCO3).
The elimination of Cd followed a very slow process for the two species studied.
The elimination rates of Cd from the mosses were not influenced by water
hardness.
INTRODUCTION
The acute toxicity and accumulation rate of Cd in the aquatic biotope
depends on many environmental factors, of which one is water hardness. Some
previous studies have examined the influence of Ca++ (Pickering and
Puia 1969, Wehr 1983, Wehr and Whitton 1983) and Mg++ (Wells and
Brown , 1990) on the accumulation of heavy metals by the aquatic mosses.
However, the present study is unique because the concentrations of Cd and water
hardness used are representative of conditions found in Quebec freshwater.
Consequently, this study investigated the accumulation andelimination rates
of Cd by Fontinalisdalecarlica and Platyhypnidium riparioides
under controlled conditions of the laboratory. The Cd concentrations were 0
(control), 0.8, 2 et 10 µg•L-1, which are similar to those
found in nature ( Goulet and Laliberté 1982). This study also examined
the influence of three different degrees of water hardness (10-15, 40-50 and
80-100 mg*L-1 as CaCO3), at constant alkalinity (80-100
mg•L-1 as CaCO3) and pH (7.30).
The objectives of this study were to evaluate the accumulation and
elimination rates of Cd by two aquatic mosses, F. dalecarlica and P.
riparioides, when exposed to three different water hardness by testing the
hypothesis that Ca++ and Mg++ compete with Cd during its
accumulation by the mosses.
MATERIALS AND METHODS
Mosses Used
These experiments were conducted using two bryophytes Fontinalis dalecarlica
Schimp. ex B.S.G. and Platyhypnidium riparioides (Hedw.) Dix., from the
Mastigouche Reserve, Quebec (46°40'N; 73°19'W).
Experimental Design
The experiments concerning the accumulation rates were carried out in a
semi-open dynamic system; a 25 L aquarium was connected by three pipes to a drum
filled with 190 L of water. Before starting the accumulation experiments, the
systems were exposed to Cd in order to saturate their surfaces (Thain 1984). The
elimination rates of Cd was followed using only the 25 L aquaria equipped with a
simple air pump circulating system, which maintained the water homogeneity.
Methods of Contamination
The nominal Cd exposure concentration in the study were 0 (control), 0.8, 2
and 10 µg•L-1 (with replicates for the 10 µg•L-1
concentration). A commercial Cd reference solution (1000 mg•L-1
of Cd ± 1% with 2% HNO3, Fisher Scientific) was used to inject
Cd in each system. Each contamination level was reproduced in three types of
reconstituted water; very soft (10-15 mg•L-1 as CaCO3),
soft (40-50 mg•L-1 as CaCO3) and hard water (80-100
mg•L-1 as CaCO3) (Table I), for a total of 15
experimental set- ups. Like it was said previously, the water hardness selected
in this study were mostly soft water because it is representative of Quebec
streams. A buffer solution of NaOH (1M) and KH2PO4 (1M)
was added tomaintain a constant pH (EPA 1975, ASTM 1987).
Exposition Schedule for Accumulation and Release
The accumulation patterns of Cd by the bryophytes was followed for 28 days.
Three samples of both mosses (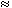 0.3 g dry
weight) were removed at intervals of 0, 1.5, 3, 6, 12, 24 h and every other day
thereafter.
0.3 g dry
weight) were removed at intervals of 0, 1.5, 3, 6, 12, 24 h and every other day
thereafter.
After the 28 days of the accumulation phase, the contaminated mosses were
immersed in Cd free water in order to determine their elimination capacity. The
sampling schedule for the elimination of Cd from the mosses was the same as that
for the accumulation phase.
Analyses of Cd in Moss and Water
The Cd measurements were made following the method described by APHA (1989).
Digestion blanks and standard additions were used to complete the quality
control scheme. Quantification limit of the instrument used (Varian SpectrAA
10/20 with graphite furnace equipped with a deuterium lamp) was 0.15 g•L-1
of Cd (three times the limit of detection).
Statistical Analysis
In order to compare the Cd accumulated by the aquatic mosses, a
bioconcentration factor (BCF) (Eq. 1), inspired from the works of Bryan (1983)
and Mouvet (1987), was computed. The percentage of elimination (%E) (Eq. 2) was
calculated from the Cd lost between the value at the beginning of the release
and the average value of the last two measured values . The Wilcoxon test for
paired-samples was used when a comparison of the BCF and the %E was carried out
on the two aquatic mosses.
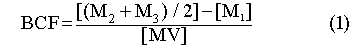
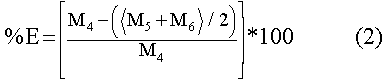
Where
| BCF |
= |
Bioconcentration factor |
| % E |
= |
Percentage of elimination |
| M1 |
= |
Mean accumulation of Cd at 0 h (µg•g-1 dry weight) |
| M2 |
= |
Mean accumulation of Cd at 672 h (µg•g-1 dry weight) |
| M3 |
= |
Mean accumulation of Cd at 624 h (µg•g-1 dry weight)
|
| M4 |
= |
Mean elimination of Cd at 0 h (µg•g-1 dry weight)
|
| M5 |
= |
Mean elimination of Cd at 624 h (µg•g-1 dry weight)
|
| M6 |
= |
Mean elimination of Cd at 672 h (µg•g-1 dry weight)
|
| MV |
= |
Mean value of Cd in water (µg•L-1 of Cd)
|
The simple linear regression values were calculated using the accumulation
or elimination rates (slope) of each system. The analysis of variance (ANOVA)
was calculated to know if the accumulation and elimination rates by the mosses
were significantly different from zero. After that, the rates were compared
using the analysis of covariance, which allows one to determine if the hardness
of the water influences the accumulation or elimination rates of Cd by aquatic
mosses. The entire statistical analysis was executed using SPSS X software
(Norusis 1983).
RESULTS
Accumulation Profile
The accumulation by the mosses in contaminated system was significant (ANOVA
p>0.05). The concentration values of Cd accumulated by the bryophytes as a
function of time are expressed as the mean of the three different subsamples
analysed independently (µg•g-1 dry weight).
There is not much variation in the controls, the small excesses observed
initially were attributed to slight initial contamination of the experimental
system. At 0.8 µg•L-1, there was a small Cd accumulation
in the mosses. At 2 µg•L-1, the Cd accumulation was
obvious. At 10 µg•L-1, the increase of the Cd
concentration in the mosses was very noticeable. The Cd concentration in the
aquatic mosses varied more at the end of the experiment, at least for soft and
hard water where a steady state conditions was partially observed. These results
were consistent with previous studies as far as the Cd absorption rate by
aquatic mosses (Claveri 1995).
Elimination Profile
The Cd elimination rate found was much slower than the accumulation rates.
The elimination by the mosses was significant (ANOVA p>0.05) and was similar
to that reported by other studies (Mouvet 1985, Mersch et al. 1993b).
During the same time span, the aquatic mosses had accumulated more Cd than
they eliminated. Since the elimination process was very slow, contamination by
Cd produced a memory effect in the mosses, a necessary requirement for the
environmental studies in nature which allows one to measure punctual and/or
sporadic contaminations (Mouvet et al. 1993).
Bioconcentration factor (BCF) and Percentage of Elimination (%E)
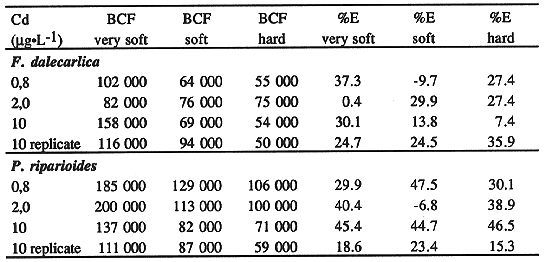
The maximum BCF(s) at 10 µg•L-1 of Cd were 158 000 for
F.dalecarlica and 137000 for P.riparioides, factors which are
very high for a 28 day study (Table I).
Table I Bioconcentration Factors (BCF) and Percentage of
elimination (%E) in Accordance with the Cd Exposure Test
For comparison, Tessier et al. (1994) calculated a theoretical BCF for two
molluscs using a two-compartment rate model. Their results showed that the
gastropod (Viviparus georgianus) and the pelecypod (Elliptio
complanata) subjected to a contamination of 10 µg•L-1
of Cd for 60 days attained a theoretical steady state BCF maximum of 35 000 and
6 000 respectively. Thus, these results show that the aquatic mosses tested are
more sensitive to the level of cd contamination of natural water and perform
outstandingly even at the very low Cd concentrations used in this study.
The BCF decreases as the water hardness increases for all concentrations
tested and for both species studied (Table I). These results demonstrate the
competition between the ions responsible for water hardness (Ca++,
Mg++) and the total accumulation of Cd by aquatic mosses. The
significant differences observed between the BCF of the two bryophytes (Wilcoxon
paired n=12 z=-2.353 p<0.05), with P.riparioides generally bioconcentrating
more Cd than F. dalecarlica was also reported by André and
Lascombe (1987).
The maximum %E were 39.4% and 47.5% for F.dalecarlica and P.riparioides
respectively (Table I). There were no differences between the %E for the two
aquatic mosses (Wilcoxon paired, n=12, z=-0.078, p=0.948). which eliminated Cd
roughly at the same rate.
Accumulation Rates
The accumulation rates and the standard errors for each system are presented
according to the Cd contamination and the hardness types in Table II. The
replicate aquaria have been combined for subsequent analysis. A covariance test
was applied to verify the influence of water hardness on accumulation rates
(Table II). The analysis shows a significant difference in the controls which is
consistent with intra-species variations. Nevertheless, the accumulation rates
for the controls were markedly smaller than those exposed to Cd contaminations.
Table II Accumulation Rates of Cd (+SE) According to the Cd
Exposure and the Hardness Type
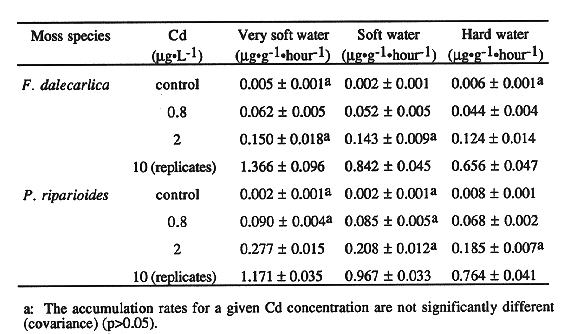
The accumulation rates for the mosses subjected to different Cd
concentrations were influenced by water hardness (Table II), and there was a
significant difference between the rates of the mosses exposed in very soft (
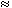 15 mg•L-1 as CaCO3)
and hard water (
15 mg•L-1 as CaCO3)
and hard water (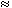 90 mg•L-1 as
CaCO3). However, cd uptake observed in soft water was often similar
to those measured in very soft and hard water It is important to mention that
the range of the water hardness selected in this study was closely related to
thoseobserved in Quebec streams. A nalogous studies performed on other organisms
used a larger range of water hardness, generally 0-250 mg•L-1
as CaCO3 ( Markich and Jeffree 1994, Wurts and Perschbacher 1994).
90 mg•L-1 as
CaCO3). However, cd uptake observed in soft water was often similar
to those measured in very soft and hard water It is important to mention that
the range of the water hardness selected in this study was closely related to
thoseobserved in Quebec streams. A nalogous studies performed on other organisms
used a larger range of water hardness, generally 0-250 mg•L-1
as CaCO3 ( Markich and Jeffree 1994, Wurts and Perschbacher 1994).
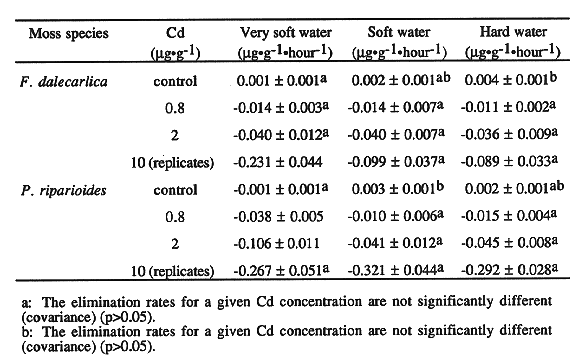
Elimination Rates
The elimination rates that were not significantly different according to the
covariance test are identified in Table III. Generally, there were no
significant differences of the elimination rates between very soft, soft and
hard water. Hence, the water hardness did not have a significant effect on the
elimination rates for both mosses.
Table III Elimination Rates of Cd (+SE) According to the Cd
Exposure and the Hardness Type
DISCUSSION
The first goal of this study was to asses the performance of aquatic mosses
as a biomonitor. The mosses accumulated Cd and demonstre the exceptional
integrator potential of this sensor. Even at low Cd concentrations (below 6.82 µg•L-1
of Cd), the observed Cd accumulation by the mosses was higher in contaminated
systems compared to the controls, which demonstrates the sensitivity of the
aquatic mosses. Even through the accumulation was very strong for both mosses
studied, P.riparioides accumulated more intensively.
The water hardness influenced the accumulation rates so that there was a
marked difference between very soft water and hard water. The observed
laboratory accumulation is considered to be greater than the accumulation
expected to be found in nature because in vivo there is a lower Cd
bioavailability due to more dissolved and suspended organic matter which
inhibits its availability (Mouvet 1985). A difference of 45 mg•L-1
as CaCO3 between soft water and hard water was not sufficient to
statistically separate the accumulation in the mosses. At smaller differences in
hardness, the accumulation differences noted between the mosses resulted from
the natural fluctuations in the intra-species variability or other surrounding
factors, such as water current (Claveri 1995), pH, alkalinity, synergy
phenomenon, among other. Note however that this difference of hardness (45 mg•L-1
as CaCO3) was estimated at a hardness range lower than 100 mg•L-1
as CaCO3 and may not hold for very hard water. Further studies in the natural
environment will be essential to establish the validity of this value.
This study was carried out to determine the effect of the water hardness on
Cd accumulation and elimination in mosses. Water hardness integrates the two
important environmental ions (Ca++and Mg++ ions) and
several studies have shown that Ca and Mg decrease heavy metal accumulation by
various organisms (Wells and Brown, 1990, Markich and Jeffree 1994), whereas
other studies used only on the protector effect of the Ca++ ions
(Pickering and Puia 1969, O'Shea and Mancy 1978 ). In hard water Ca++
and Mg++ ions play a protective role as they compete with Cd ions.
This competition should result in the availability or the preference of the
plants for Ca++ and Mg++ ions, thus decreasing the
number of available sites for Cd. Cadmium, a non- physiological element, takes
advantage of its high complexation capacity with the surface sites of the cell
wall and of its chemically similar properties to other physiological elements,
such as Ca and Mg, to pass through the plasmic membrane.
The results of the elimination phase showed that Cd elimination was very
slow. Hence the mosses showed a significant memory effect for Cd. Because of
this memory effect, these mosses represent a good choice of sensor for
environmental studies.
REFERENCES
- American Society for Testing and Materials (ASTM) "Annual book of ASTM
standards". Philadelphia (11.04) (1987).
- American Public Health Association (APHA). "Standard methods for the
examination of water and wastewater". 19 th edition, APHA, Washington
(1989).
- B. ANDRÉ and C. LASCOMBE. "Comparaison de deux traceurs de la
pollution métallique des cours d'eau: les sédiments et les
bryophytes". Sci Eau 6(2):225-249 (1987).
- G. W. BRYAN. "Brown seaweed, Fucus vesiculosus and the gastropod
Littorina littoralis, as indicators of trace-metal avaibility in estuaries".
Sci Total Environ 28:91- 104 (1983).
- B. CLAVERI. "Les bryophytes aquatiques comme traceurs de la
contamination métallique des eaux continentales. Influences de différents
paramètres sur l'accumulation des métaux et développement
d'un Module d'Intégration de la Micropollution (M.I.M.)". Thèse,
Centre de Recherches Écologiques, Université de Metz, Metz, France
(1995).
- Environnemental Protection Agency (EPA). "Methods for acute toxicity
tests with fish, macroinvertebrates, and amphibians". Springfield, National
technical information service (No EPA-660/3-75-009) (1975) .
- M. GOULET and D. LALIBERTÉ. "Métaux: contamination du
milieuaquatique au Québec méridional. Ministère de
l'Environnement, Service de la qualité des eaux, Québec".
Envirodoq, ND-83-0017 (1982).
- M. L. KINKADE and H. E. ERDMAN. "The influence of hardness components
calcium and magnesium in water on the uptake and concentration of cadmium in a
simulated fresh water ecosystem". Environ Res 10(2):308-313 (1975).
- Le Conseil National de Recherches du Canada (CNRC). "Les effets du
cadmium dans l'environnement canadien". Le Conseil National de Recherches
du Canada, publication du secrétariat de l'environnement, 16744, Ottawa
(1979).
- S. J. MARKICH and RA. JEFFREE. "Absorption of divalent trace metals as
analogues of calcium by australian freshwater bivalves: An explanation of how
water hardness reduces metal toxicity". Aquat Toxicol 19:257-290 (1994).
- J. MERSCH, E. MORHAIN and C. MOUVET. "Laboratory accumulation and
depuration of copper and cadmium in the freshwater mussel Dreissena polymorpha
and the aquatic moss Rhynchostegium riparioides". Chemosphere
27(8):1475-1485 (1993).
- C. MOUVET. "The use of aquatic bryophytes to monitor heavy metals
pollution of freshwater as illustrated by case studies". App Limnol 2,
Toxicity (Verein Limnol.) 2420-2425 (1985).
- C. MOUVET. "Accumulation et relargage de plomb, zinc, cadmium, chrome
et cuivre par des mousses aquatiques en milieu naturel et au laboratoire.
Rapport de l'agence de Rhône-Méditerranée-Corse, 31 rue
Jules Guesde, F-69310 Pierre-Bénite (1987).
- C. MOUVET, E. MORHAIN, C. SUTTER and N. COUTURIEUX. "Aquatic mosses
for the detection and follow-up of accidental discharges in surface waters".
Water Air Soil Pollut 66:333-348 (1993).
- M. J. NORUSIS. "Introductory statistics guide, SPSSX".
McGraw-Hill, Illinois. (1983).
- T. A. O'SHEA and K. H. MANCY. "The effect of pH and hardness metal
ions on the competitive interaction between trace metal ions and inorganic and
organic complexing agents found in waters". Water Res 12:703-711 (1978).
- D. C. PICKERING and I. L PUIA. "Mechanism for the uptake of zinc by
Fontinalis antipyretica" Physiol Plant 22:653-661 (1969).
- L. TESSIER, G. VAILLANCOURT and L. PAZDERNIK. "Comparativestudy of
cadmium and mercury kinetics between the short-lived gastropod Viviparus
georgianus (Lea) and the pelecypod Elliptio complanata (Lightfoot), under
laboratory conditions". Environ Pollut 85:271-282 (1994).
- J. E. THAIN. "Effects of mercury on the prosobranch mollusc Crepidula
fornicata: acute lethal toxicity and effects on growth and reproduction of
chronic exposure". Mar Environ Res 12:285-309 (1984).
- J. D. WEHR and B.A. WITTON. "Accumulation of heavy metals by aquatic
mosses. 2: Rhynchostegium riparioides". Hydrobiologia 100:261-284 (1983).
- J. M. WELLS and D. H. BROWN. "Ionic control of intracellular and
extracellular Cd uptake by the moss Rhytidiadelphus squarrosus (Hedw.) Warnst."
New Phytol 116:541- 553 (1990).
 15 mg•L-1,
soft
15 mg•L-1,
soft 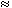 45 mg•L-1 and hard
water
45 mg•L-1 and hard
water 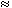 90 mg•L-1 as CaCO3)
was measured while maintaining the alkalinity and pH constant during the provide
duration here. The Cd accumulation by the aquatic mosses was rapid, showing the
potential of accumulation and sensitivity of this biomonitor. Even when the
actual Cd concentration in the water was low (concentrations <0.15 to 6.82 µg•L-1,
the uptake of Cd was very fast and mostly linear. Accumulation rates of Cd were
significantly different when the mosses were in very soft (
90 mg•L-1 as CaCO3)
was measured while maintaining the alkalinity and pH constant during the provide
duration here. The Cd accumulation by the aquatic mosses was rapid, showing the
potential of accumulation and sensitivity of this biomonitor. Even when the
actual Cd concentration in the water was low (concentrations <0.15 to 6.82 µg•L-1,
the uptake of Cd was very fast and mostly linear. Accumulation rates of Cd were
significantly different when the mosses were in very soft (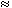 15 mg•L-1) as compared to hard
water (
15 mg•L-1) as compared to hard
water (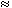 90 mg•L-1 as CaCO3).
The elimination of Cd followed a very slow process for the two species studied.
The elimination rates of Cd from the mosses were not influenced by water
hardness.
90 mg•L-1 as CaCO3).
The elimination of Cd followed a very slow process for the two species studied.
The elimination rates of Cd from the mosses were not influenced by water
hardness.