Lisa Clement and Goran Jovanovic
Department of Chemical
Engineering
Oregon State University
Corvallis, OR 97331
ABSTRACT
Oregon State University WERC-Task-Force has developed a unique and innovative engineering approach toward cleaning up liquids, sludges, and contaminated soil type wastes. The extensive contamination by heavy metals, hydrocarbons, chlorinated biphenyls, and in some cases radio nuclides has presented a great challenge for developing effective engineering methods for remediation of soil, sludges, and contaminated liquids. Existing technologies often fall short of productive remediation due to stringent requirements and high expectations while trying to comply with free release CERCLA regulations. This work represents an effort to enhance our engineering options in dealing with a variety of clean-up situations and requirements.
As a result, the use of a magnetically stabilized fluidized bed (MSFB) is envisioned to remove particularly targeted or an array of contaminants from liquids and sludges containing mixtures of different above mentioned contaminants. MSFB incorporates high mass transfer and high conversion rate characteristics of packed beds, in addition to excellent characteristics of fluidized beds like: low pressure drop, and the ability to process solids. A magnetically stabilized fluidized bed is the addition of a magnetic field to the traditional fluidized bed. Fluidized particles contain a ferromagnetic material which can be magnetized while in the magnetic field. Magnetized ferromagnetic particles create inter-particle magnetic forces which act to control the dynamic behavior of fluid and solids for the purpose of increasing or controlling the intensity of multiphase contact. Magnetic field strength, fluid velocity, particle composition, percentage of solids in the sludge, and fluid viscosity are all adaptable operating parameters. The MSFB has been shown to have robust operating characteristics and to have enhanced mass transfer capabilities up to 75% while efficiently remediating sludges of 20-30% solids.
The MSFB technology is based on the custom made fluidizing particles containing ferromagnetic material and additional active substances like adsorbents and catalysts. Ferromagnetic particles act as platforms for a multitude of active substances that can be tailored toward a variety of specific applications/tasks. For example, our research indicates that the use of OSU-Sorb-2R particles can be successfully implemented for simultaneous adsorption and catalytic decomposition of hydrocarbons in sludges.
INTRODUCTION
Due to the wide array of chemicals present in contaminated site areas, no single technology is sufficient for treating the liquid, sludge and soil to comply with free release CERCLA regulations. In addition, technologies for soil, sludge, and liquid treatment are difficult to combine. For example, present technologies for treating soil include soil vapor extraction (SVE) and soil washing but do not apply to liquid and sludge. Soil vapor extraction works on the premise that organics are removed by utilizing their vapor pressures (McCann,1994). However, simultaneous presence of metals and/or radioactive material makes any of these methods ineffective in achieving a satisfactory overall solution. However, our research effort is oriented toward conception of a comprehensive but simple solution by designing a practical engineering platform capable of implementing a series of integrated unit operations to achieve CERCLA approved treatment levels.
MAGNETICALLY STABILIZED FLUIDIZED BED
The Magnetically Stabilized Fluidized Bed (MSFB) is one of the most recent and novel chemical engineering developments in the area of fluid-solid contacting operations. It combines the best characteristic of the fluidized bed, like low pressure drop and the ability to handle solids without clogging due to sediment buildup, with excellent efficiency of the fixed bed in mass transfer between fluid and solid particles.
Fluid-particle mass transfer in fluidized beds is a very important, often
the limiting, transport parameter for operations such as adsorption, ion
exchange, drying, and evaporation. It is well known that in a conventional
fluidized bed, particles are subject to three macroscopic forces: the
gravitational force Fg, the drag force Fd, and the
buoyancy force Fb. As soon as the drag force, which is measured by
the pressure drop, across the bed,
![]() p,
balances the other two forces the particles are fluidized.
p,
balances the other two forces the particles are fluidized.
In a MSFB, however, an additional force is created by applying the magnetic
field to the ferromagnetic particles. The magnetic field magnetizes particles
containing ferromagnetic material and creates magnetic forces between the
particles. The induced particle-particle forces tend to bring the particles
together, which results in a decrease of the bed porosity, e. Additional
macroscopic forces are also created within MSFB which must be overcome by the
fluid drag force. Hence, the fluid velocity through the bed must increase just
to compensate these new macroscopic forces. Consequently, in a MSFB we are able
to maintain lower bed porosity while increasing the superficial fluid velocity,
uo. This means that the relative velocity between the fluid and the
particles, the interstitial velocity uint = uo/![]() , is
substantially increased; hence, the mass transfer coefficient between the
fluidizing particles and the fluid must also increase. This increase of the mass
transfer coefficient in a MSFB is 40% to 75% over that in a conventional
fluidized bed (Fig. 1).
, is
substantially increased; hence, the mass transfer coefficient between the
fluidizing particles and the fluid must also increase. This increase of the mass
transfer coefficient in a MSFB is 40% to 75% over that in a conventional
fluidized bed (Fig. 1).
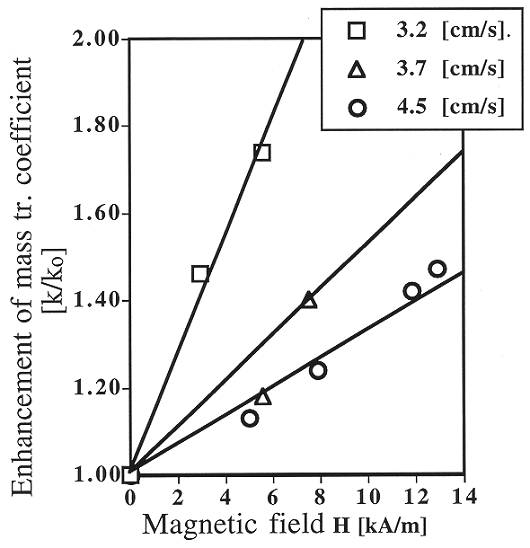
Fig. 1. Enhancement of the mass
transfer coefficient as a function of magnetic field intensity.
We found that the following correlation excellently predicts the enhanced mass transfer coefficient in a MSFB (Al-Mulhim, 1995; Al-Mulhim and Jovanovic, 1995).
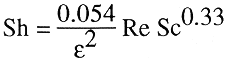 (3)
(3)
In the correlation, the bed porosity, e, is shown as a function of the magnetic field intensity and ferromagnetic properties of particles, as derived from the original experiments of Honorez (1994), and Jovanovic and Honorez (1994)
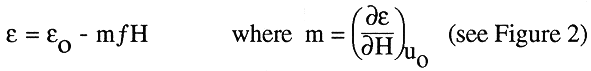 (2)
(2)
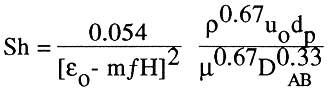 (3)
(3)
also,
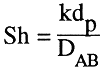 (4)
(4)
finally,
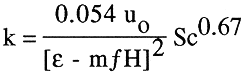 (5)
(5)
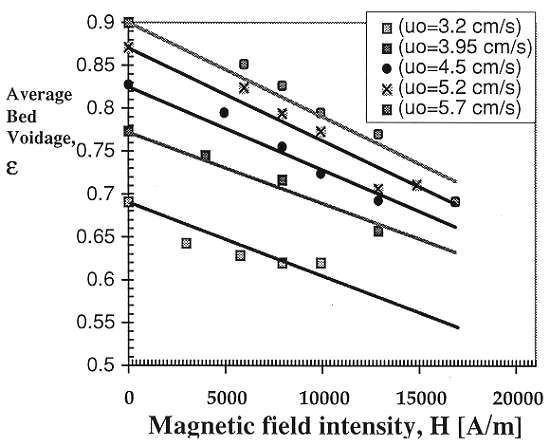
Fig. 2. Bed voidage as a function of
magnetic field intensity.
FLUIDIZATION PARTICLES
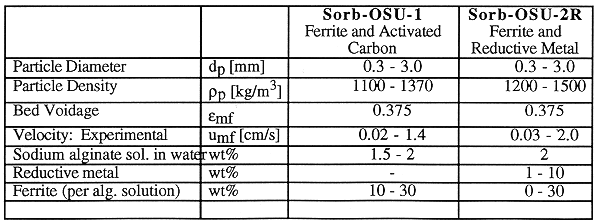
The basic fluidization particles used in MSFB are composite ferromagnetic particles which are made from the mixture of sodium alginate and ferromagnetic powder. Further, the content of the particles can be modified to fit our purposes. In the process of adsorbing the contaminants in a slurry, activated carbon, ion exchange resin, elemental iron or zinc are added into the alginate particles to form OSU-Sorb-1 and OSU-Sorb-2R types of particles. The activated carbon serves as an adsorption site for the organics while ion exchange resin collects metal ions. Elemental (zero valent) zinc or iron serve as electron donors in the process of reductive dechlorination. The individual bead characteristics are shown in Table I.
TABLE I Particle Characteristics
The preparation and production of the alginate particles is a simple, straightforward process. The design schematic for preparation and production of the particles is shown in Fig. 3. Typically, a solution of 2% sodium alginate in water, ferromagnetic powder, and either granulated activated carbon, ion exchange resin, zinc powder or iron powder is mixed in a vessel. The solution is pumped by a gear pump through a nozzle. As shown in Fig. 3, air is supplied through the outer cylinder to shear off the particles from the tip of the nozzle. This helps to ensure desired size of particles. Once a particle is sheared off, it drops into a 1-2 M solution of calcium chloride. The alginate exchanges two Na+ ions for a Ca2+ which immediately creates the protective "skin" of the particle. The particles are left in solution for a short period of time which is a clear distinction from other known particle manufacturing processes (Klein et. al., 1983). The core of the particle is usually left in liquid state. Both the alginate and the activated carbon or ion exchange have strong adsorptive properties for organic and polyvalent metal ions. The flexibility in the amount of time that the particles spend in the calcium chloride provides us with the capability to control polymerization of the alginate and indirectly the diffusivity of different molecular compounds through the bead.
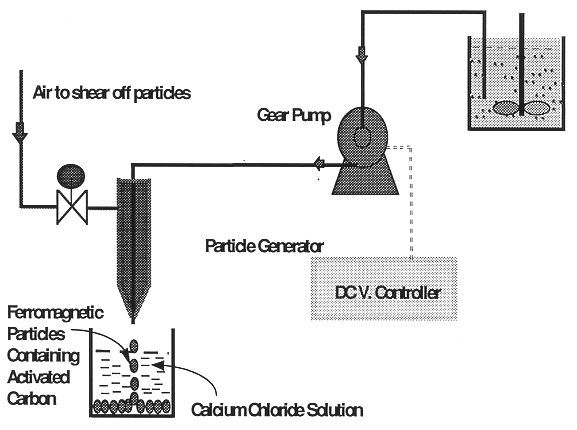
Fig. 3. Particle
production schematic diagram.
DECHLORINATION
Another specific application of the MSFB technology is envisioned in the area of selective dechlorination of chlorinated hydrocarbons in liquids and sludges. Previous research has focused on degrading chlorinated hydrocarbons utilizing metal surfaces of magnesium, zinc, tin and iron. The process of dechlorination by metal in aqueous solutions follows the process of chemical corrosion of the metal by the chlorinated compound (Schlimm and Heitz, 1966).
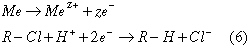
While zinc, tin, and iron successfully dechlorinated several compounds, magnesium reactions were found to be overwhelmed by the competing reaction of oxidation by water. (Boronina et al. 1995). One example of a proposed mechanism for iron on trichloroethylene is as follows (Matheson and Tratnyek, 1994).
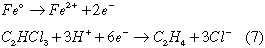
Currently, several researchers are investigating the chemistry behind reductive dechlorination of chlorinated hydrocarbons using metal surfaces. These developments include a wide range of commonly found environmental contaminants including tetrachloroethylene (PCE), trichloroethylene (TCE), chloroform, lindane, pentachlorophenol and polychlorinated biphenyls (PCBs). The perspective metal surfaces include Zno, Feo or specially prepared bi-metalic surfaces ( Pd-Fe, Ni-Fe) depending upon the particular task of dechlorination.
According to research done by Tratnyek and Matheson (1994), iron sequentially dehalogenates an aliphatic chlorinated hydrocarbon such as carbon tetrachloride with an initial pseudo-first-order in substrate. Hence, the Feo metal system quickly degrades (Fig. 4B) the heavily chlorinated hydrocarbon and gradually slows with each sequential dechlorination step. Other experiments include the dechlorination of lindane and chloroform in which over 99% conversion was achieved in 1-2 hours using a Zn surface (Fig. 4A) (Schlimm et. al., 1996). Unfortunately, straight chain conjugated compounds such as PCE require significant time before degrading and produce low concentrations of DCE isomers which are persistent (Gillham and O'Hannesin, 1994).
Recently, the Pd-Fe bimetallic system has been used to tackle aromatic chlorinated hydrocarbons such as pentachlorophenol and PCBs. One important characteristic of Pd-Fe systems is its ability to thoroughly dechlorinate all compounds present without leaving chlorinated side-products. For example, the Pd-Fe surface dechlorinated all PCB congeners within the Aroclor 1260 and Aroclor 1254 mixtures at ambient temperatures (Gritini et. al, 1995). This reaction took between 5 and 10 minutes with biphenyl as the only final product. Also, complete dechlorination was reported for 2,3-dichlorophenol in the presence of a Pd-Fe surface as seen in Fig. 4C (Agrawal, 1997).
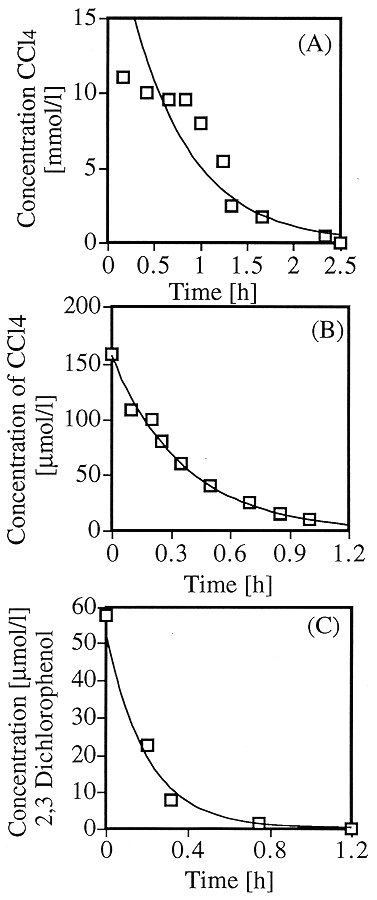
Fig. 4. a) Degradation of CCl4
by Zn dust in water Boronina et al. (1995); b) Degradation of CCl4
by Fe dust in water, Matheson and Tratnyek (1994); c) Degradation of 2,3
Dichlorophenol in water by bimetallic Pd-Fe catalyst Agrawal (1997).
Preparation of the Pd-Fe surface is created through a simple process of exposing clean Feo to a solution of K2PdCl6 for a few minutes. Recently, X-ray photoelectron spectra of the palladium-iron bimetallic surface was performed (Muftikian et. al, 1996). This analysis included the important aspect of restoring the Pd-Fe surface to its original reactivity. Fortunately, the Pd-Fe surface was nearly completely restored through washing with 3M HCl and no loss of Pd was detected during any experiment. This is a favorable indication of recycling the metal for further dechlorination in the MSFB.
MSFB APPLICATION
Importantly, these researchers have worked at near groundwater temperatures (15-25[°C]) and at low concentrations of these environmental contaminants. These conditions are in agreement with the aims of our technology towards the treatment of contaminated soil and sludge materials. Further, we opt to design a platform upon which to combine the dechlorination chemistry with our carrier alginate beads. The metal powders used can be easily incorporated into our alginate carrier. The alginate solution itself is approximately 98% water. Diffusion coefficients for solutes in alginate beads have been reported as having the same magnitude as aqueous diffusivities (Oyaas et. al., 1995). Therefore, it is expected that the diffusion into the alginate bead and subsequent reaction would be on the same order of magnitude as in the batch experiments described in literature.
There are several avenues in which to incorporate the dechlorination chemistry into the MSFB engineering process. One application involves impregnating the carrier alginate beads with powdered metal. Inside the MSFB, these beads will adsorb and dechlorinate the chlorinated hydrocarbons from sludge/slurry environment. Another possibility involves a two-step process. Alginate beads containing activated carbon within the MSFB can adsorb chlorinated hydrocarbons from the sludge system. These beads can then be transferred to a "clean" environment with a metal surface such as Pd-Fe for complete dechlorination. A twist on this disposal method involves using an Feo surface with elevated temperatures to promote dechlorination.
CONCLUSION
The MSFB technology provides a novel and effective approach for treating mixed waste in liquid and sludge/slurry systems. The flexibility of the fluidized particles to contain a variety of active substances allows them to be tailored for multiple applications. Specifically, OSU-Sorb-2R particles promote the dechlorination of chlorinated hydrocarbons through the addition of zero-valent metal powder. Importantly, the link between dechlorination chemistry and our MSFB engineering process gives us flexibility in dealing with a wide variety of environmental contaminants. Through dechlorination, this advancement reduces the chlorinated compounds to a collection of non-chlorinated hydrocarbons which could be further remidiated through incineration. Based on documented research in this area, we are developing several of these proposed methods.
REFERENCES