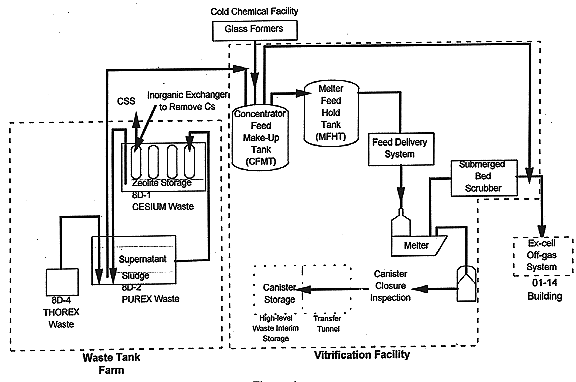
Fig. 1. WVDP Process Flowsheet.
Vijay Jain and Steven M. Barnes
West Valley Nuclear
Services Company, Inc.
West Valley, NY 14171-0191
ABSTRACT
The immobilization of the high-level wastes (HLW) into a stable and nondispersible form using vitrification was successfully initiated at the West Valley Demonstration Project (WVDP) in July 1996. Since then, several batches of the HLW stored in a carbon steel tank (8D-2), each approximately 2,000 gallons, have been transferred from the Waste Tank Farm (WTF) and vitrified into a stable canistered waste-form. Tank 8D-2 in the WTF contains a single HLW that is a blend of washed PUREX sludge, Cs-loaded zeolite, and neutralized THOREX waste. The vitrification process is designed to meet the Waste Acceptance Product Specifications (WAPS) to strictly control HLW processing from its transfer to its eventual disposal in a geological repository. The HLW transferred from Tank 8D-2 is mixed with the glass-forming chemicals and fed to a joule-heated melter at 1150°C. The glass is then poured into stainless steel canisters. After the canisters have cooled, they are welded, decontaminated, and stored on site for eventual disposal. To ensure process reliability and that the canistered waste-form complies with all WAPS requirements, the batch-to-batch variability of the transferred waste and the variability associated with the sampling and analysis were analyzed and compared with the WAPS. In the following sections, the vitrification process is discussed based on the process data collected to date, and an assessment is provided of the canistered waste-form properties relative to the WAPS requirements.
INTRODUCTION
The high-level radioactive waste (HLW) at the West Valley Demonstration Project (WVDP) was generated by the commercial reprocessing of 640 tons of reactor fuel. At the inception of the WVDP, two wastes were stored in underground tanks. The majority, 2,200 m3, was neutralized PUREX waste. Another 40 m3 of partially processed THOREX waste was also stored. The PUREX waste was washed to minimize the sodium and sulfate salts to be vitrified. The original supernatant and wash solutions were decontaminated using a zeolite ion-exchange process to remove the cesium. Prior to the start of radioactive operations, the THOREX and zeolite wastes were blended with the washed PUREX sludge in Tank 8D-2. The volume of the waste at the start of radioactive operations was approximately 1,000 m3.
PROCESS OVERVIEW
The vitrification process can be separated into three phases: tailoring the waste slurry by adding glass-forming chemicals to achieve the desired composition, melting the waste slurry and casting the glass into canisters, and closing the canistered waste-forms. The overall process is shown in Fig. 1 and each phase is discussed below.

Fig. 1. WVDP Process Flowsheet.
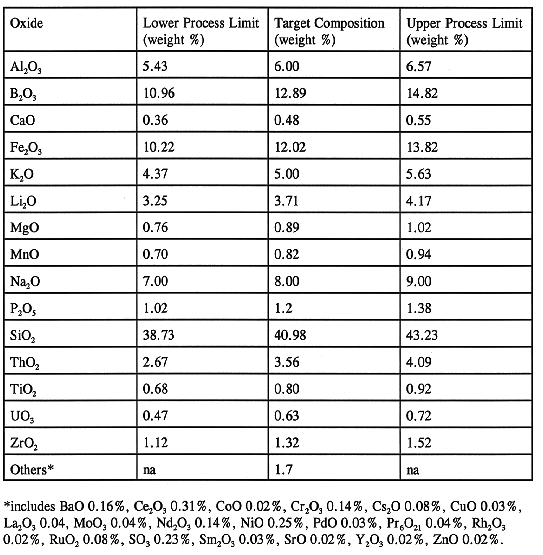
The initial step in the vitrification process is the pumping of a batch of HLW slurry from Tank 8D-2 to the concentrator feed makeup tank (CFMT) in the Vitrification Facility (VF). Approximately 100 batch transfers will be required to process the HLW inventory. At the CFMT, the waste is sampled and concentrated by evaporating water from the slurry. Chemicals are added to the CFMT, based on the waste sample analysis results, to adjust the waste slurry to the target glass composition. The target glass composition and its acceptable process region are shown in Table I. Following confirmation that the desired slurry chemistry has been achieved and that the feed is adjusted to the target reduction/oxidation (redox) ratio by the addition of sugar, the feed is transferred to the melter feed hold tank (MFHT).
Table I WVDP Target Glass Composition
The second phase is initiated by pumping the slurry from the MFHT to the melter. At the melter, the remaining water is evaporated from the slurry and the waste fuses with the glass-forming chemicals thereby producing the target waste-form composition. Molten glass is periodically poured from the melter into 3 m tall by 0.6 m diameter stainless steel canisters. After the glass solidifies, the canisters are routed for closure, decontamination, and on-site storage.
In the final process stage, the canisters are closed by welding a lid to the canister. The welding technique is an automated, autogenous, pulsed-gas tungsten arc welding process. (1) Following a visual quality verification inspection of the closure weld, the canisters are decontaminated using an acidic, cerium-based, chemical oxidation procedure. (2) After decontamination, the canistered waste-forms are stored on site until they can be shipped off site to a federal repository for storage or disposal.
The following sections discuss efficiency and control of the process to produce a consistent glass waste-form. As of September 1996, nine radioactive batches have been processed through the vitrification system producing 27 canisters.
TRANSFER OF HLW TO THE VF
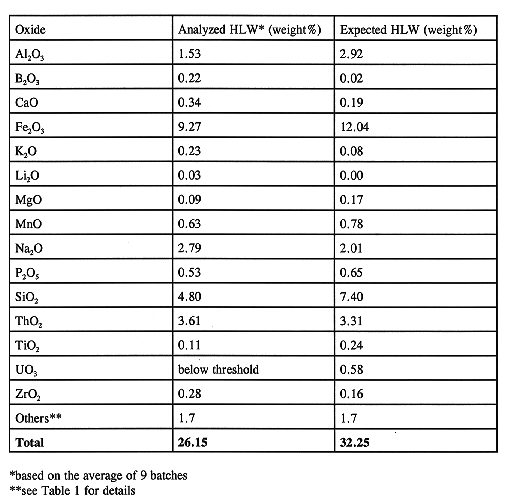
The process starts with a transfer of a known amount of waste from Tank 8D-2 to the CFMT. The HLW transfer is made on the top of the existing heel (about 1,000 liters) and the recycled solution from the aqueous process off-gas scrubber. Table II shows the amount of waste transferred to the CFMT in the last nine batches, the Sr-90 and Cs-137 activity, and the amount of glass produced from each transfer. Sr-90 and Cs-137, along with their daughters Y-90 and Ba-137m, are the major radioactive species in the waste. They account for more than 98% of the total activity. The amount of waste transferred for each batch depends on the available volume in the CFMT for a new transfer and the expected volume of the glass-former chemical addition. The composition of the HLW transfers to date have been very consistent. This is shown in Fig. 2 where the major components (as oxides) have been ratioed to ThO2. Even though the HLW transfers have been very consistent in composition, the transferred waste has fallen short of the expected composition of the HLW in Tank 8D-2. The expected waste composition, based on previous analysis and records, is shown in Table III along with the current composition. This discrepancy has been attributed to a less-than-anticipated suspension of solids in Tank 8D-2 due to the failure of one of the long-shafted mixing pumps. Installation of a replacement pump in Tank 8D-2 is planned during early FY 1997 to improve mixing in the tank. (3) Note that the variance in HLW composition has no impact on the production of the target glass composition. The required adjustment to the composition is done by the addition of glass-formers as discussed under Glass Former Preparation.
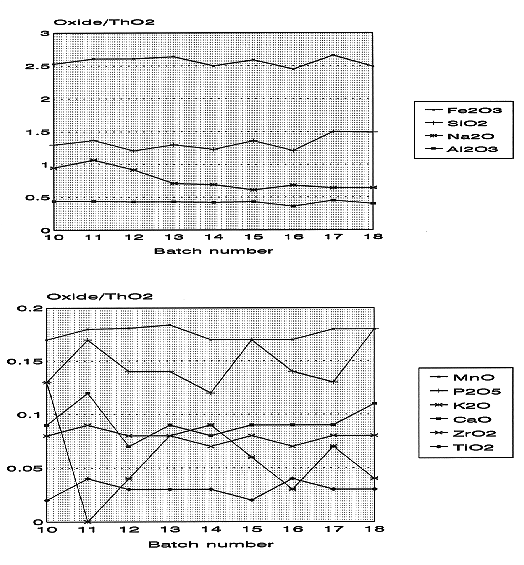
Fig. 2. Tank 8D-2 composition normalized to ThO2.
Table II Volume and Selected Activity of HLW Transferred for Vitrification During Each Batch and the Amount of Glass Produced
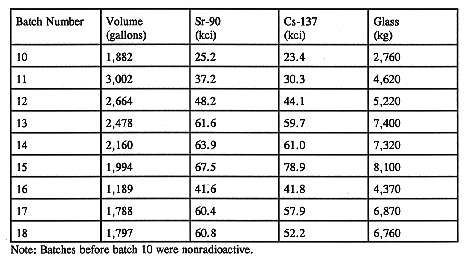
Table III Analyzed and Expected HLW Composition
CONCENTRATION OF WASTE
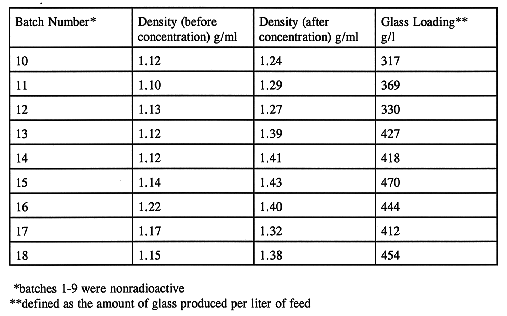
The amount of HLW that could be transferred or the maximum glass that could be produced from a waste transfer depends upon the concentration potential of the wastes in the CFMT. The concentration potential is enhanced in the CFMT by the addition of sodium metasilicate as a deflocculating agent. The pre-concentration, post-concentration waste density and the final glass loading are listed in Table IV for each batch. The glass loading is defined as the amount of glass produced per liter of feed. Efforts have been made to maximize the concentration of the HLW in the CFMT since the beginning of radioactive operations. So far, a maximum glass loading of 470 g/l has been attained in the CFMT without any processing problems. This has enabled enhancing the batch size from an initial 6,000 kg of glass (3.2 canisters) to 7,500 kg of glass (4.0 canisters) per batch of HLW transfer without any slurry handling equipment problems. The improved batch size is a direct result of WVDP's ability to concentrate and process concentrated feed through the vitrification process lines and pumps.
Table IV HLW Density Before and After Concentration and the Final Glass Loading
GLASS-FORMER PREPARATION
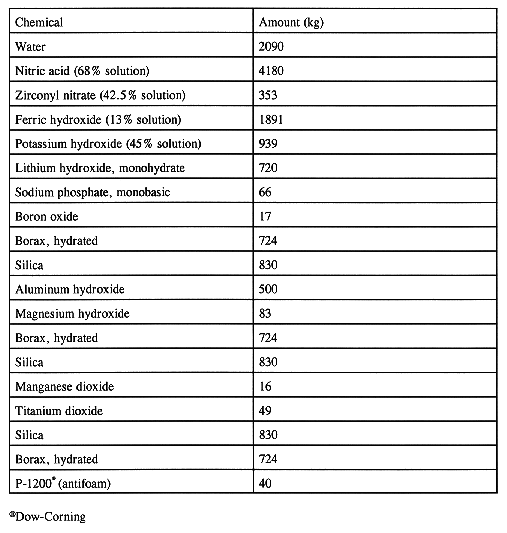
Based on the chemical analysis of the CFMT contents, the total amount of glass-forming chemicals required to produce the WVDP target glass composition is calculated. Table V shows the amount of glass-former oxides needed for a typical batch of 7,500 kg of glass as based on the HLW analysis and the target glass composition. The glass-formers are prepared in a nonradioactive facility and added in a specific sequence to minimize glass-former preparation time. A typical addition sequence is shown in Table VI. Nitric acid is added to the glass-formers to control the pH of the feed. The target nitrate concentration is 0.42 gram NO3 per gram of glass. The feed pH control is needed to improve the rheological properties of the feed. The completed batches are sampled and analyzed prior to transfer to the MFHT.
Table V Typical Glass-former Oxides for a 7,500 kg Glass Batch
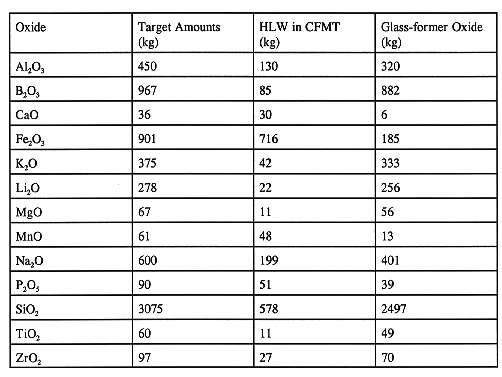
Table VI Typical Glass-former Chemical Addition Sequence
WASTE-FEED SLURRY
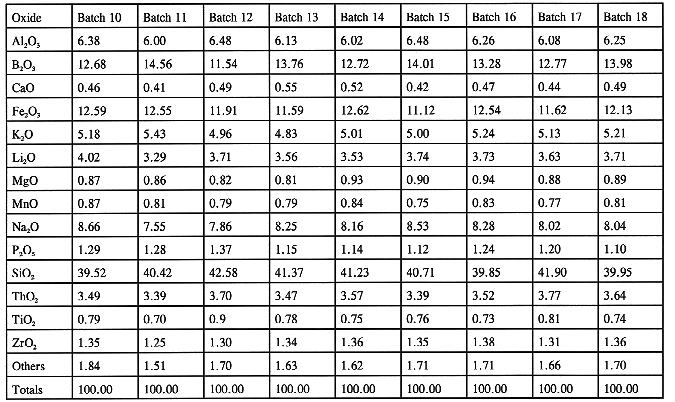
Waste-feed slurry is the final mix of all chemical species needed to produce the target waste-form. The chemical analysis of this slurry is the key holdpoint for approval to transform the final slurry mix into glass. The Waste Qualification Report (WQR)4 requires that for the feed batch to be of acceptable composition for making the waste-form, it should fall within the processing region shown in Table I. Table VII shows the compositions of the waste-feed batches processed to date. The final compositions of all the batches processed to date are within the processing region of the target glass composition. Even though composition of all the batches shown in Table VII fall within the acceptance range, the composition of batches 11, 12, and 13 were outside the acceptance region at the first chemical analysis. WQR states that if the composition of feed falls outside the acceptance region, a new set of samples will be taken and analyzed, and then the analyses will be grouped with the previous set to redetermine the chemical composition of the slurry. Furthermore, if the batch is still not acceptable the required chemicals will be added and the contents will be resampled and reanalyzed. Batches 11 and 12 both required chemical shimming to pass; while batch 13 was acceptable after reanalysis.
Table VII Analyzed Slurry Feed Batch Composition (weight %) (batches 1 through 9 were nonradioactive)
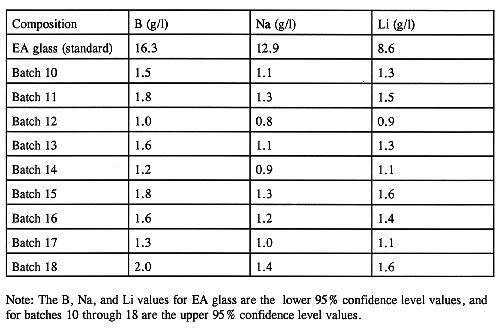
In addition, WQR specification 1.3 requires that before the final mix is approved for making glass, the mean of the normalized release rates for B, Na, and Li in the feed (as estimated by the prediction equations in Ref. 4) is at least two standard deviations below the mean normalized release rates for B, Na, and Li in the Environmental Assessment (EA) glass using the product consistency test (PCT). Table VIII shows the estimated upper bound 95% confidence limit values for the normalized PCT release rates for B, Na, and Li in the feed, along with the lower bound 95% confidence limit values for the normalized release rates for the EA glass. The data indicate that the release rates are not only acceptable but are well below the lower limit of the EA glass.
Table VIII Normalized Leachate Concentration Estimates on the Final Waste-feed Batches (batches 1-9 were nonradioactive)
SUGAR ADDITION TO THE WASTE-FEED SLURRY
The final step in feed preparation is to estimate the amount of reductant to be added to the feed. The reductant addition is necessary to provide proper redox conditions in the melter. The impacts of these conditions are discussed in detail in Ref. 5. The amount of sugar needed is calculated using an empirical relationship shown below:
IFO = NO3(1-TS)/TOC
where IFO is the index of feed oxidation, NO3 is the total nitrate concentration, TS is the fraction of total solids in the feed, and TOC is the total organic carbon concentration. A target IFO of 3.0 was used to estimate the amount of sugar required to process the feed between the Fe2+/Fe3+ ratios of 0.01 and 0.20. Table IX shows the total nitrates, total organic carbon, percent total solids, and calculated IFOs for radioactive batches to date. In several batches, the analyzed IFOs were different than the target value of 3.0, indicating either an oxidizing IFO (>3.0) or a reducing IFO (<3.0). The observed variations are attributed to sampling and analytical errors. Based on these results, a safe operating region for the target IFO has been established between 2.5 and 3.8.
Table IX Index of Feed Calculation Data

GLASS PRODUCTION
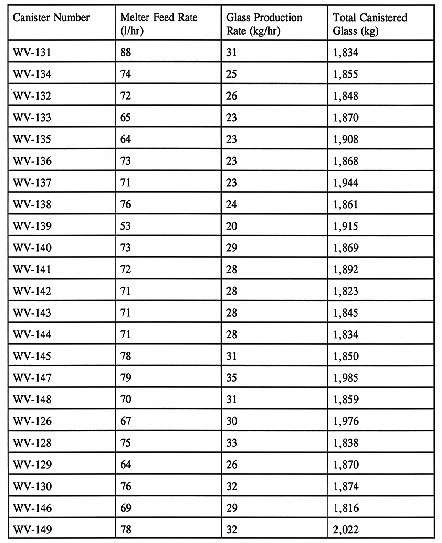
The sugared waste-feed slurry is transferred to the MFHT and mixed with the heel of the previous batch. The combined slurry is then fed to the melter. The feed rate depends upon the melting characteristics of the feed. Higher total solids and sugar concentrations promote higher feed rates, while higher water and nitrate concentrations decrease feed rates in the melter. An optimum balance is maintained between total solids, nitrates, and sugar to maximize the feed rate while maintaining the Fe2+/Fe3+ratio within the specified range. Table X shows the comparsion between the feed rate and the glass production rate. The data indicate that beyond the 9th canister, the glass production rate has steadily increased. The increase is attributed to the higher total solids in the feed. The amount of glass poured in each canister is also shown in Table X.
Table X Average Feed Rates and Glass Production Rates
SUMMARY
In summary, the WVDP has successfully demonstrated the conversion of HLW into a stable borosilicate glass waste-form. As of September 1996, 10% of the canistered waste-form has been produced and all waste acceptance criteria have been met. The entire process is anticipated to be completed in June 1998. The canistered waste-form is stored in the Interim Storage Facility on site until a decision is made on a final HLW repository.
REFERENCES