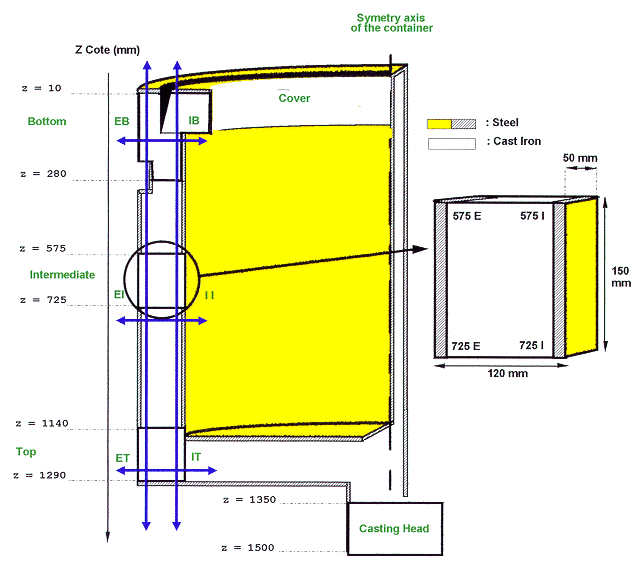
Fig. 1. Comparative study of cast
iron following different axis.
M.H. Plouzennec, D. Feron, R. Atabek
CEA (Commissariat à
l'Energie Atomique)
BP 6 - 92265 Fontenay-aux-Roses Cedex (France)
M. Tachon
CEA (Commissariat à l'Energie Atomique)
bp 171 - 30207 Bagnols-sur-Cèze Cedex (France)
J. Corcos
ANDRA (Agence pour la Gestion des Déchets
Radioatifs)
1-7 rue Jean Monnet, 92265 Chatenay-Malabry Cedex (France)
ABSTRACT
This paper presents the results of a qualification program developed to characterize the performances of a cast iron container submitted to subsurface disposal environmental conditions with a particular attention to the corrosion resistance experiments.
Corrosion tests are performed at 40°C in a synthetic water representative of a concrete interstitial pore water.
The two materials of the container, cast iron and steel are studied separately.
Pitting corrosion sensibility is evaluated by means of cycling polarization in the anodic field following ASTM G61 norm. Crevice corrosion specific tests are also performed.
Results demonstrate that the corrosion rates of cast iron are low in concrete water (less than 10 µm.y-1) and localized corrosion and crevice corrosion could be considered as negligible.
A qualification program is developed to characterize the performances of a cast iron container submitted to a subsurface disposal environmental conditions.
Containers are manufactured from very low contaminated metallic wastes melt in the UDIN arc furnace plant located at Marcoule site. Cast iron is poured in specific steel moulds to elaborate separately body and cover of the container. All the planned and systematic steps of the process are implemented within a quality system and containers are submitted to quality control tests.
The qualification program includes the main following parts :
In order to perform these different types of experiments, a container has been cut following the main axis in two parts, one for coupons, the other one for archives. In a quarter of container, coupons have been machined in the most sensitive points of the casting. These points are supposed to be located in a shrinkage region or in a region susceptible to include bubbles. Three main regions in the container itself are so defined: Top, Intermediate and Bottom. Two complementary regions are also defined : cover and casting head. In order to be more precise in the qualification protocol the exterior and the interior of the container are differentiated. A general view of container sampling is represented in Fig. 1. Thus the container can be qualified following the two vertical axes and the three horizontal axes. These experiments will allow also to determine if the corrosion behavior of the casting head is representative of the behavior of the whole container.
GENERAL CORROSION RESISTANCE
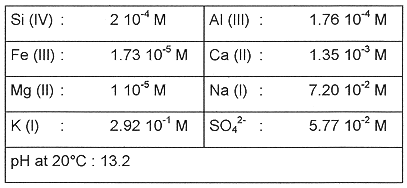
One of the main objective of the corrosion study is to characterize the casting homogeneity and the cast iron behavior in a synthetic solution representative of a concrete water at a temperature of 40°C (Table I). The two materials of the container are studied separately.

Fig. 1. Comparative study of cast
iron following different axis.
This general study includes general corrosion and localized corrosion experiments.
TABLE I Concrete Interstitial Pore Water Composition
In a first step, intensity potential curves are performed to evaluate the corrosion intensity likely to occur. In a second step, other coupons are immersed in the concrete water during two months. Meanwhile this period free corrosion potential and polarization resistance are measured. At the end of this experiment weighting are performed. In the Table I the results obtained with the different methods are collected.
TABLE II Summary of the Main Results Obtained with
Cast Iron in Concrete Water (E: potential; I: current ;
![]() e:
corrosion rate )
e:
corrosion rate )
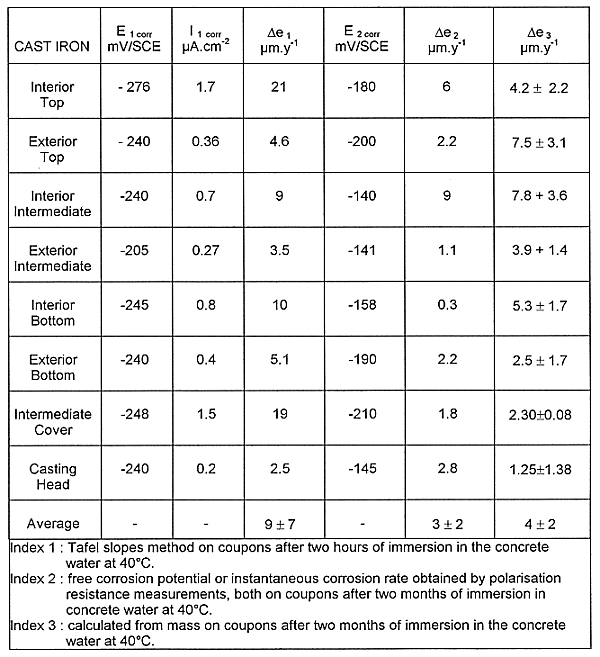
All the intensity potential curves can be superposed and show the same electrochemical response of cast iron for all the points of the container. The corrosion rates obtained at the intersection of Tafel slopes after two hours of immersion are higher than those obtained after two months of immersion and correspond to coupons not protected by deposits.
In fact after two months of immersion coupons are covered with a white non uniform deposit on their surface. This product has been identified as being Ca5Si6O16(OH)2 or Ca5Si6O17(OH)2 by means of x-ray diffraction analysis
A descaling has been requested to obtain significant weight losses. It appears that the corrosion rate of cast iron in term of thickness loss is relatively homogeneous, inferior to 10µm.y-1 (Table II).
The presence of these products may explain that the instantaneous corrosion rates measured by polarization resistance is relatively lower than those calculated from weighting. The monitoring of the instantaneous corrosion rate shows a relative homogeneity of the behavior of cast iron from different points. The instability of the instantaneous corrosion rate is represented on the Fig. 2. It can be pointed out that an evaporating problem in an electrochemical cell has occurred an increase of the instantaneous corrosion rate (pink curve on Fig. 2). This increase has been quickly compared by adding demineralized water up to the normal level. The values of free corrosion potential at the end of the period confirm the homogeneous behavior of cast iron from different points of the container. These have increased to a more noble field along the two months confirming the overall protective effect of the deposit.
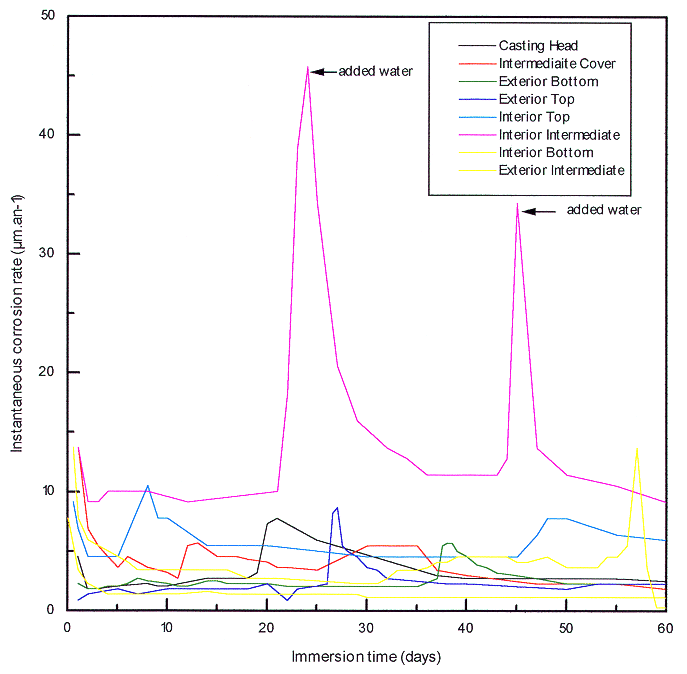
Fig. 2. Instantaneous corrosion rate
of cast iron in concrete water at 40°C.
LOCALIZED CORROSION
Pitting Corrosion
The sensibility to pitting corrosion has been evaluated by means of an electrochemical test, the ASTM G 61 norm. An intensity potential curve has been recorded under potentiodynamic control in the anodic field, first to the increasing potentials then to decreasing potentials. The evaluation of the resistance to pitting corrosion is performed by determining the values of the pitting initiation potential (Er) and of pitting repassivation potential (Ep). In fact three cases can be observed depending on the solution and the immersion time.
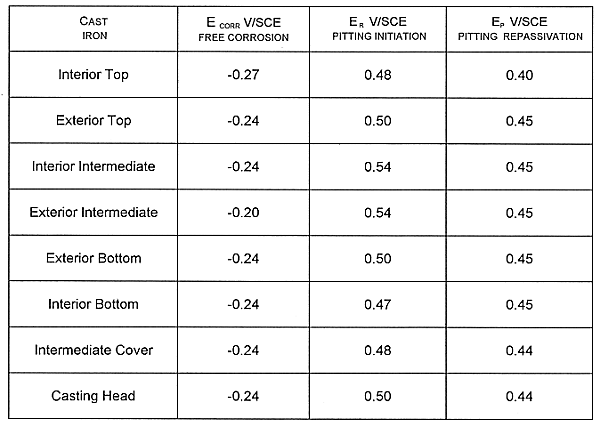
The results obtained from the different points of the container are summarized in the Table III.
It can be noticed that the metal potential stays lower than the pitting repassivation potential. No pitting will be promoted and all the existing pitting will not propagate anymore. The passivity of the cast iron is stable. The values of potentials Er , Ep are very far from the corrosion potential that corresponds to a good resistant to pitting corrosion. The values of pitting initiation potentials and pitting repassivation potentials are close. This means that the polarization does not occur any significant hysteresis and the system might be considered as reversible.
When we superposed all the polarization curves performed from the different points of the container the obtained bundle confirms the homogeneity of the cast iron. This is particularly clear on the Fig. 3 representing the behavior of cast iron following an exterior vertical axis of the container. It could be noticed also that potentials measurements increases are not far closed to those corresponding to water composition. It means that what has been interpreted as pitting passivation potentials could only correspond to potentials at which water decomposition occurs.
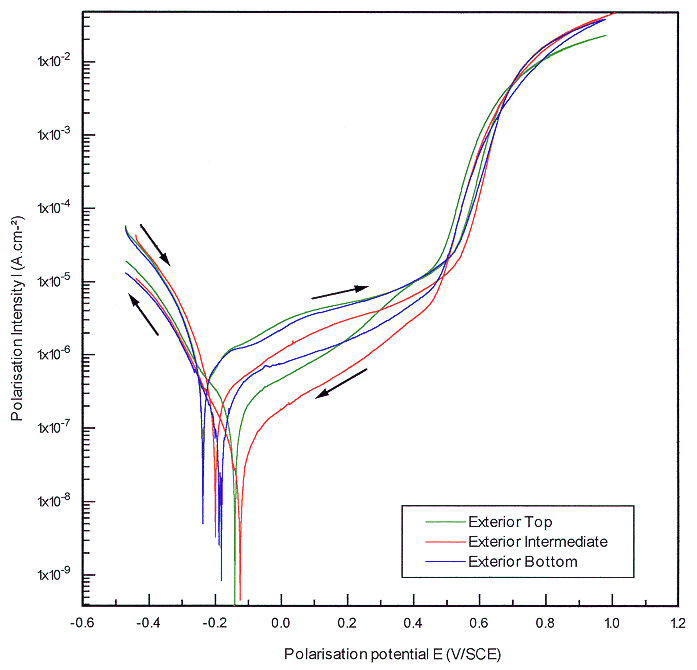
Fig. 3. Sensibility to localized
corrosion of cast iron in concrete water at 40°C. Comparison of the
behavior of cast iron following a vertical casting axis.
TABLE III Localized Corrosion Test in Concrete Water
Interstitial Corrosion
This interstitial corrosion test is very close to a crevice corrosion test. It is performed with coupons simulating a rather bad adhesion between steel and cast iron. The mounting allows to reproduce phenomena occurring in corners or interstices at the interface between steel and cast iron. A little sheet of cast iron and an other one of steel separated with a teflon chock are joined with a teflon screw. The teflon chock lets a 1 mm space between cast iron and steel around the screw. After two months of immersion in concrete water an hazardous very slight rusty deposit has been observed between steel coupons and PTFE chock or cast iron coupons and PTFE chock. After descaling, observations by means of a microrugosimeter have set some pitting of 20 to 40 µm deep for cast iron and one of 100 µm deep for steel. The mass variation average of cast iron corresponds to a thickness loss of 5 µm.y-1, for an sheet surface of 16cm2. The surface examinations and mass variations lead to conclude that the interstitial corrosion might be considered as negligible for all the points of the container.
GALVANIC COUPLING
We have elaborated the following hypothesis that the concrete water might percolate through interstice between steel and cast iron in the case of bad adhesion between the two metals and lead to a galvanic corrosion. In order to be representative of the container, coupons of steel were sampled in the points facing theses machined for the cast iron. The intensity potential curves obtained for different points of the steel mould are rather close as presented on Fig.4 By superposing steel and cast iron polarization curves it appears that the galvanic coupling effect, if any, would be beneficial for cast iron, reducing the corrosion intensity by a factor of ten. This corrosion intensity would be minimized to 0.1 µA.cm-2, corresponding to an instantaneous corrosion rate of roughly 1 µm.y-1.
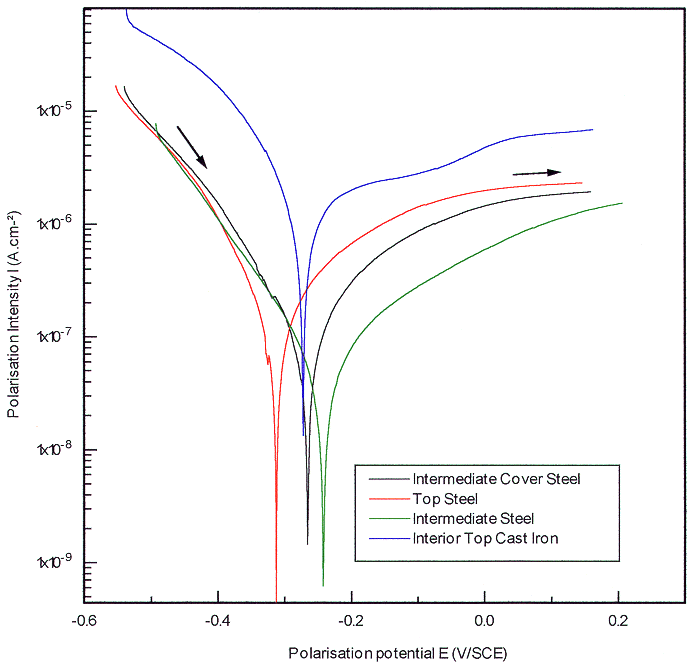
Fig. 4. Galvanic
coupling effect between steel and cast iron.
CONCLUSION
This study has set the characterization of cast iron in a synthetic solution representative of a concrete water. The cast iron presents an. homogeneous behavior concerning the general corrosion and the localized corrosion considering the horizontal and vertical axes and the interior and exterior of the container. The general corrosion rate of cast iron is very low, inferior to 10 µm.y-1 and the localized corrosion might be considered as negligible. The cast iron is very little sensitive to pitting corrosion and to interstitial corrosion. All the experiments of general and localized corrosion have shown a rather good homogeneity of the cast iron in all the different axes of the container but also for the cover and the casting head. The behavior of casting head seems to be representative of the overall behavior of the container. All these experimental conclusions might lead to foreseen a reasonable durability of container under disposal environmental conditions.