T.Sasaki, K.Suzuki
Oarai Nuclear Research Center
JGC Corporation
2205 Naritacho Oaraimachi
Ibaraki 311-13 Japan
Phone: 81-29-266-3311
M.Kiyomoto, Y.Karasawa
Kayatone Research Laboratory
Nippon Kayaku Co. Ltd.
219 Iwahana Takasaki
Gunma 370-12 Japan
81-273-46-2411
ABSTRACT
This study investigates a combination process consisting of denitration and cement solidification for the treatment of DOE tank wastes which contain large amounts of sodium nitrate and low-level radioactivity. In the treatment process, sodium nitrate in DOE tank waste is first converted to another sodium salt during denitration, and the sodium salt is finally solidified with an alkali activated slag cement(AASC). Sodium sulfate in several sodium salts appears to be the most feasible for the combination process, because the sodium sulfate content in the form solidified with AASC was nearly equal to the content of tank waste in the vitrified glass products.
This paper reports results of denitration and AASC solidification, and discusses the basic process of the combination.
INTRODUCTION
Treatment of DOE tank wastes with large amounts of sodium nitrate/nitrite and low-level radioactivity is one of the important issues in the remediation program of DOE sites. (1) Two technologies, cement grouting stabilization and glass vitrification have been evaluated for the treatment. The cement grouting is a simple method and many studies about the treatment of DOE tank wastes have been carried out. (2) However, the leachability of nitrate/nitrite in the stabilized cement forms was not always low enough to demonstrate safe disposal.
Glass vitrification has the capability to strongly stabilize radionuclides, but the maturity of technology for treatment of DOE tank wastes is not advanced, compared with the cement grouting technology. Operation performance, maximum content ofwastes, volatility of Cs, S, Cl and others, characteristics of vitrified product, off-gas treatment of NOx and the operating life of melter equipment should be confirmed by further studies. (3)
In order to offer an alternative technology for the treatment of tank wastes, JGC studied the application of solidification of alkali activated slag cement(AASC) supplied by Nippon Kayaku Co. Ltd. In the first place, the slurry solution of the simulated DOE tank wastes was directly solidified by AASC, and it was shown that the AASC has an effective ability to stabilize tank wastes (4). Moreover, the waste form had low leachability for nitrate/nitrite and radionuclides. The obtained values of Leaching Index satisfied a target value required in a safety evaluation which was concerned with the migration of nitrate/nitrite from a certain disposal site in Hanford to Colombia river (5).
However, nitrate/nitrite in the final waste forms should be removed as much as possible, to originally solve the problem of contamination in drinking water. Therefore, another advanced technology based on AASC solidification was investigated. The technology is a combination process which consists of both the denitration and the solidification of AASC.
This study reports experimental results of denitration which sodium nitrate/nitrite in tank wastes converts to another sodium salts and of SSAC solidification for several sodium salts, and then discusses a basic process system for the combination.
Selection of Sodium Salt
In the combination process, sodium nitrate/nitrite first converts to a sodium salt during the denitration, shown here. The produced sodium salt is finally solidified by AASC solidification. Then, the selection of sodium salt is a key point to establish the process system.

In case of vitrification for the DOE tank waste, the glass product will be able to finally incorporate 20wt% of Na2O (3). The waste content corresponds to 55wt% of sodium nitrate in the tank waste. Therefore, the target weight content of each sodium salt for the AASC solidification was estimated to be equal to 55wt% of sodium nitrate. Anhydrous Na2SO4, NaBO2, Na2SiO3, etc. were selected. Table I shows the results. A sodium salt with smaller target weight content is better than others, because the waste form with small content of sodium salt provides a much more stabilized product with high compressive strength, no swelling and low leachability.
TABLE I Solidification of Various Sodium Salts by
AASC
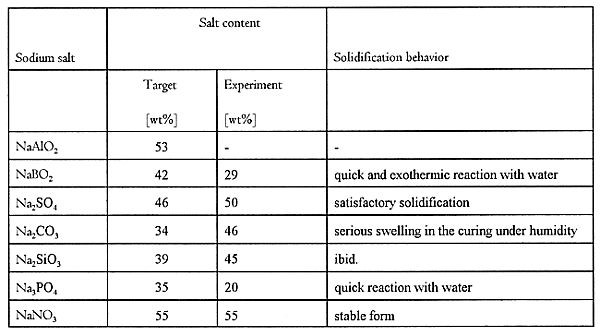
Sodium metaborate(NaBO2), sodium sulfate(Na2SO4), sodium carbonate(Na2CO3), sodium silicate(Na2SiO3) and sodium phosphate(Na3PO4) were selected in this preliminary evaluation, because these target salt contents are smaller than 55wt% of sodium nitrate.
Sodium carbonate(Na2CO3)
There were many studies about the denitration of sodium nitrate. It is known that the denitration in the presence of sugar, urea and other carbon compounds proceeds at a low temperature, 300-400°C to finally obtain Na2CO3(6). Then, the conversion to anhydrous Na2CO3 is a proven process.
Powder of anhydrous sodium carbonate was treated with AASC. Table I shows the result. The salt was hardened with a high weight content, but the solidified product underwent serious swelling within 1-2 weeks even in the curing under humidity. Even in the waste forms containing smaller weight content than the target, the swelling always appeared, due to the conversion reaction of anhydrous Na2CO3 to hydrated salt,Na2CO3.9H2O.
Consequently, the waste form of Na2CO3 with the target content was not available.
Sodium sulfate(Na2SO4)
Denitration
A direct production of sodium sulfate during the denitration of NaNO3 was carried out in the presence of sulfur powder. The sulfur melted and volatilized at T=150°C, and no conversion to Na2SO4 was occurred.
Alternative Treatment:
To obtain sodium sulfate, NaNO3 first converts to anhydrous Na2CO3 by the denitration mentioned above, and then Na2CO3 is directly neutralized with sulfuric acid solution. The solution containing Na2SO4 is dried to obtain a pulverized anhydrous Na2SO4. These processes have been already proven.
AASC Solidification:
The anhydrous sodium sulfate content of the solidified form was 50wt% and satisfied the target(Table I). Fig. 1 shows a relationship between the weight content and the compressive strength. Moreover, there were no swelling in water immersion.
Sodium sulfate is a suitable salt for the combination process, although the intermediate conversion step to Na2CO3 is needed.
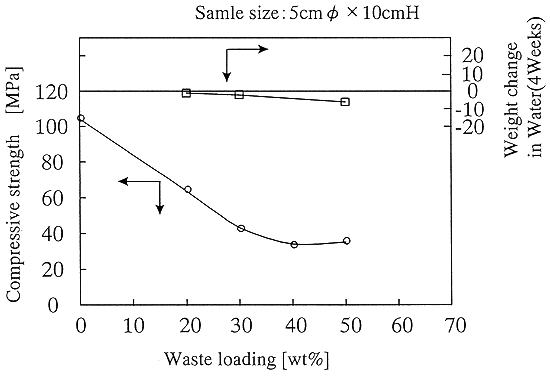
Fig. 1. Compressive strength and
weight change in water immersion for sodium sulfate product.
Sodium Silicate(Na2SiO3)
Denitration:
In the presence of silicon powder the denitration of sodium nitrate was carried out. Fig. 2 illustrates spectra of TG and DTA analysis curves of the denitration reaction. The denitration begins at T=650°C, and there is a strong exothermic reaction between 730°C and 750°C. The chemical compositions formed in this denitration were analyzed by X-ray diffractometer. Shown in Fig. 3, Na2SiO3 is formed by the direct reaction of sodium nitrate with silicon at T=750°C. At lower temperature, the product of sodium silicate contains the residue of NaNO3. At 850°C another silicate salt, Na2Si2O5 mixes with Na2SiO3. The temperature will increase higher than 700°C, to completely denitrate NaNO3in the presence of silicon.
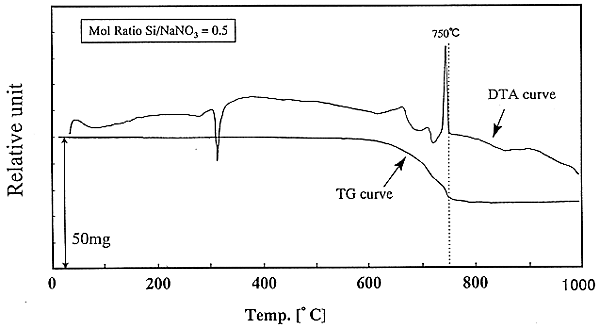
Fig. 2. TG and DTA
curves in reaction of NaNO3 with Si.
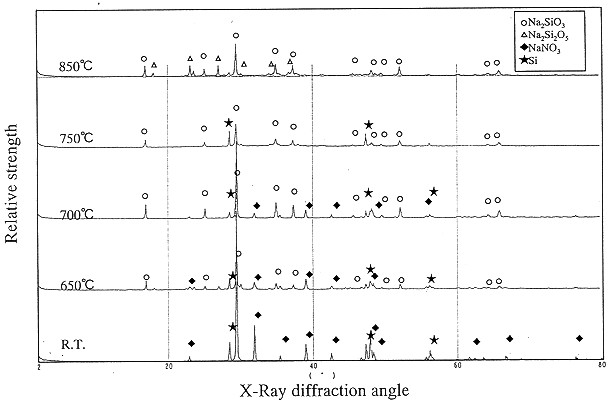
Fig. 3. XRD analysis
of compositions in reaction of NaNO3 Si.
AASC Solidification:
The solidification behavior of sodium silicate was complicated (Table I). Fluidity of the mixture of AASC with anhydrous Na2SiO3 decreases and stiffens during the solidification at T=20°C, and it was difficult to mold the mixture. Hardening at a higher temperature, about 50°C, normally proceeds, however, the solidified form was completely broken by serious swelling in the curing under humidity. In the present experiment of solidification, an appropriate condition of AASC solidification for sodium silicate was not found.
The temperature condition of the salt conversion to Na2SiO3 is much higher that of sodium carbonate, and also the AASC solidification is not able to form a good product. Therefore, sodium silicate will be excluded.
Other Salts
Sodium metaborate(NaBO2) and sodium phosphate(Na3PO4) were solidified with AASC at a small weight content. It was difficult to stabilize anhydrous sodium metaborate by AASC, because anhydrous NaBO2 reacts with water quickly and exothermically. The salt content was only 29wt%, about two-third of the target content.
In case of Na3PO4, the anhydrous salt also reacts with water quickly, and the content in the solidified form was only 20wt%, less than the target content.
Retention of Radionuclides in Waste Forms Solidified with AASC
Leaching test was carried out to determine the retention of radionuclides in the waste form containing 50wt%Na2SO4. Table II summarizes results of Leachability Index. The value of LIX was higher than a target value LIX=7.5 in the demonstration of safe disposal (5). Therefore, radionuclides will be effectively fixed in the waste form.
TABLE II Leachability of Radionuclides in Sodium
Sulfate form with AASC

Basic Concept of Combination Process
The feasibility study mentioned above comprehensively suggests that sodium sulfate appears to be the best salt for the combination process of denitration and AASC solidification for DOE tank wastes containing mainly NaNO3/NaNO2.
A basic concept of the combination process was discussed. Reference (6) reported that the simulated DOE tank wastes are denitrated in the presence of sugar at T=350°C, to produce anhydrous sodium carbonate. Then, this denitration technology would basically apply to the salt conversion of sodium sulfate. To convert the sodium carbonate to sodium sulfate, the produced Na2CO3 would be dissolved in water, and then neutralized with sulfuric acid. The solution containing sodium sulfate should be evaporated and pulverized in a drier to obtain the powder of anhydrous sodium sulfate. The powder is directly solidified with AASC. Finally the total flow of process is shown as following;
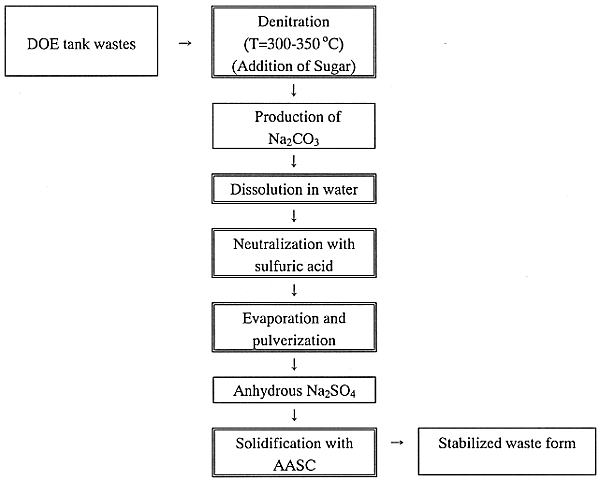
In this process concept, the denitration of DOE tank wastes to convert to Na2CO3, the AASC solidification of Na2SO4 and the pulverization to produce anhydrous Na2SO4 are in a proven stage of R&D and/or are proven technologies. Therefore, the combination process offered in this feasibility has a preferred ability to treat DOE tank wastes, though a few studies would be further done.
CONCLUSIONS
This feasibility study evaluates an application of the alkali activated slag cement solidification to the treatment of DOE tank wastes with the denitration of sodium nitrate.
REFERENCE