
Fig. 1. Containers and debris as
placed in the test pit.
Brett Campbell and Dale Timmons
Geosafe Corporation
James Brower
BNL
ABSTRACT
Pilot-scale treatability testing of the In Situ Vitrification (ISV) technology was performed on a simulated pit in the chemical/animals area of the Brookhaven National Laboratory (BNL) site. The testing was conducted as a part of an evaluation of feasible alternatives for remediating the Chemical/Animal Pits (Pits) and the Glass Holes (Holes) within OU-1 at BNL. This paper presents the results of the pilot-scale testing on the simulated pit and an assessment of full-scale remediation of the site using the ISV process. The findings are pertinent to DOE buried waste applications throughout the Weapons Complex.
A total of 31 Pits and 20 Holes have been identified by non-intrusive means at the BNL site. The Pits were active from the late 1950's to 1966. The materials disposed in the Pits consisted primarily of laboratory chemical wastes and animal carcasses used for research. The bulk of the materials is believed to be acids and bases although other chemical species are suspected to be present in the Pits; however, no known records exist. It is estimated that 1,540 cubic meters of chemical- and short-lived, low level radioactive-contaminated animal carcasses were dumped in the Pits. The Glass Holes were active from 1966 to 1981 and were used for the disposal of rinsed laboratory glassware and chemical containers. Disposed materials reportedly included acids, bases and other assorted laboratory chemicals. A test excavation of one of the holes revealed significant quantities of intact bottles containing unknown liquids. The excavation also revealed the presence of small quantities of drums ranging from 5 to 55-gal in size.
EXTENDED SUMMARY
Based upon the findings of the test excavation, a simulated pit was designed for pilot-scale ISV testing. The purpose of the test was to evaluate the effects of inclusions (partially liquid-filled bottles and miscellaneous debris) on the treatment performance of the ISV technology. The test pit was designed to be representative of the actual pits and holes based upon the findings of the test excavation. Sampling and monitoring of the process was performed prior to, during, and after treatment to fully evaluate the effectiveness of the ISV treatment process.
The primary challenge to processing of this site by ISV was posed by the presence of liquid-bearing sealed containers. Thus, the test was designed to confirm Geosafe's belief that the buried waste materials could be treated without adverse effects on the safety and efficiency of processing. In addition, a study of the general soil chemistry indicated that the site soils are low in monovalent alkali cations (Na+, Li+ and K+), which as a general rule, need to be within the 2 - 5 wt% range for efficient ISV processing. Thus, slight adjustment to the soil chemistry was made to raise the alkali content into the ideal range. The addition of alkali material to the simulated pit material only also permitted the evaluation of whether the shape of the resulting melt could be controlled by selective adjustment of the soil.
Evaluation of the test results provided the following results:
Evaluation of potential alternatives for the site is being performed based upon the following criteria: 1) protection of human health and the environment, 2) compliance with ARARs and other criteria, advisories, and guidance, 3) long-term effectiveness and permanence, 4) reduction of toxicity, mobility, or volume through the treatment, and 5) short-term effectiveness. Geosafe believes that the test results indicate ISV processing promises the maximum possible attainment of these criteria.
The conclusions of this test are significant in regards to the potential application of the ISV technology to other DOE buried waste applications. Further evaluations of the test results are continuing in regards to the selection criteria mentioned above.
INTRODUCTION
This paper presents the results of a pilot-scale treatability test using the In Situ Vitrification (ISV) technology which was performed at Brookhaven National Laboratory (BNL), located on Long Island, NY. The test was performed on a simulated burial pit containing sealed containers and miscellaneous debris in June, 1996.
Historical BNL activities produced various types of laboratory wastes from the late 1950s up to 1981. The materials were placed in either of two locations referred to as the Chemical/Animal Pits and the Glass Holes (CMP 1996).
The Chemical/Animal Pits (Pits) were initially excavated with a crane and clamshell shovel. These unlined Pits were then used for the disposal of laboratory chemicals and animal carcasses. It is believed that most of the chemicals disposed in the pits were acids and bases. The only specific chemicals described as being disposed are nitric acid and sulfuric acid, although other chemicals are presumed to have been disposed of in the Pits. It is reported that 238,350 kgs of animal carcasses were disposed in the pits. Individual Pits are expected to be up to 5-m deep and from 2 to 7-m in diameter. Thirty-one Pits have been identified, three of which may be contaminated with radiological waste.
The Glass Holes (Holes) were used for disposal of rinsed laboratory glassware and chemical containers. The Holes were believed to contain similar types of waste chemicals as those expected in the Pits; however, it is not certain that the Holes contain animal carcasses. Twenty Holes have been identified. They are approximately 3 to 3.7-m in diameter and vary from 2 to 5.8-m in depth based upon geophysical mapping data. No significant levels of radionuclides are present within the Glass Holes.
The Pits and Holes are known to possess numerous containers holding various quantities of different chemicals. In an effort to quantify the number and types of containers and their contents, a test pit (TP 43) was excavated in 1994 and the containers removed and characterized. It was reported that an estimated 6% of the volume of the Pits is occupied by intact glass bottles with varying quantities of contents. An additional 15% of the volume of the Pits is reportedly occupied by other debris". Other debris is described as broken glass, remains of metal containers and remains of laboratory instruments. Based upon the test pit inventory, the remaining 79% of the Pit volume is expected to be soil. It is believed that the Pit contents are stratified with alternating layers of laboratory waste and soil.
TEST DESCRIPTION
A simulated test pit was prepared by first excavating a hole with rough dimensions of 1.4-m square by 1.4-m deep. The pit was excavated with a trench extending to the east to allow the placement of instrumentation leads into the test area.
Prior to beginning assembly of the test pit, the chemistry of the native soil was adjusted (fluxed) to promote efficient melting. The surface soils at the site contain approximately 1.8 wt% Na2O. The soil to be melted was mixed with Na2CO3 in a 55-gal drum to achieve a concentration of Na2O in soil of 4 wt%. This fluxed soil was then used only in the area that would be melted (area inside the simulated pit bounds). Surrounding soils were not fluxed with additional sodium so that it could be determined if flux addition could be used to shape the melt and prevent excessive overmelting into unfluxed regions. The ability to control the melt shape via soil fluxing can have economical benefits by limiting the amount of uncontaminated soil that is melted.
Construction of the simulated test pit was performed by creating four different regions each 10 inches in depth. Each region was then divided into an inner and outer zone (Zone 1 & 2). These zones, which varied in radius, were sized based upon the expected vitrified mass dimensions.
Chemical compounds were placed in the test pit to simulate radioactive materials and volatile organic compounds (VOCs) that may exist within the Pits and Holes at the BNL site. BNL personnel placed containerized perfluorocarbons in the test pit to act as worst-case surrogates for VOCs. This test used four different perfluorocarbons distributed throughout the test cell.
Detection of perfluorocarbons emitted during testing was performed in the region below the test volume, within the off-gas hood, in the soil surrounding the test volume, and in the ambient air in the vicinity of the test area. Analysis of the tracer tests were performed by BNL personnel and presented in a separate report (Dietz et al. 1996).
To simulate the presence of radionuclides, four surrogates were included in the test pit. The surrogates chosen included cesium (Cs), neodymium (Nd), rubidium (Rb), and strontium (Sr). Five hundred ninety grams of CsCl in a 500 ml poly container was placed in the bottom center of the pit. All other surrogate compounds were mixed with the soil and spread vertically within the center area of all four regions in approximately equal quantities.
A total of 236 bottles ranging from 1.8 ml to 237 ml were added to the four regions (see Fig. 1). In addition, four tin cans were placed at a depth of 50 to 75 cm. All of the bottles used in the test were filled with 10 volume percent of either acetone or an aqueous mixture of water and acetone. The cans were filled with 20 volume percent of either acetone or the aqueous solution. The cans and bottles placed into the test volume contained 1,212 ml of liquid and 8,995 ml of void space. All of the bottles were capped either with plastic or phenolic-type lids and sealed in an attempt to prevent leakage into the test pit prior to treatment.

Fig. 1. Containers and debris as
placed in the test pit.
To simulate miscellaneous debris that may be present in the pits, approximately 95 l of crushed household debris was placed into the test volume (see Fig. 1). The debris consisted of broken glass, crushed metal (aluminum, tin, and some steel), and crushed plastic containers.
Instrumentation within the test volume consisted of type K thermocouples to monitor the location of the melt and type C thermocouples to determine the melt temperature.
The pilot-scale ISV unit was used to conduct the treatability test. The system consists of a power supply, a containment hood, an off-gas treatment and water collection system, and a data monitoring and storage system. The power system utilizes a 30 kW Scott-Tee transformer for converting three phase primary input power to a balanced two phase secondary output. The transformer is equipped with 12 voltage taps. Power for this test was supplied by a mobile 150 kVA generator due to the lack of utility supplied electrical service.
Off gases generated during the test were monitored via three sampling ports on the off-gas line. The off-gases were sampled during three different periods during the test for the following parameters:
Environmental Protection Agency (EPA) methods presented in the Title 40 Code of Federal Regulations, Parts 53-60, Appendix A; and in EPA document EPA- 600/8-88-085 titled "Guidelines for Stack Testing of Municipal Waste Combustion Facilities", and in Test Methods for Evaluating Solid Waste, SW-846 were used to evaluate the air emissions.
Temperatures in the soils surrounding the molten volume, in the molten mass, and of the off-gas at various locations were monitored and recorded via a temperature recorder.
Once preparation of the test cell was complete and the hood assembled and put in place, the electrodes were fed into the soil to a 2.5-cm depth. Prior to laying the starter path, a 5-cm layer of sand was placed over the treatment zone. This layer of sand provided a consistent layer of even grain size material within which to lay the starter path. The starter path was then laid. The starter path is placed in a 2.5-cm thick "X" and square pattern to provide a conductive pathway between the electrodes. This allowed for the initial conduction of current between the electrode pairs.
The zone to be vitrified was covered with a 5-cm thick insulation blanket with small gaps around the electrodes for venting. The insulation promotes subsidence of the molten zone and improves the efficiency of the process. This technique is also used in large-scale operations.
TEST RESULTS
The pilot-scale treatability test was conducted on June 24 - 26, 1996. Initiation of the melt and gradual power up to a maximum operating level of 25 kW was accomplished in approximately 4 hrs. The vitrification process proceeded to the target depth of 107 cm as indicated by thermocouple readings. The total test duration with power applied was 47.25 hrs.
A total of 1143 kWh of energy was consumed in melting the test volume. The average power level of this test was 24.3 kW (1143 kWh/47.25 hr). Assuming the surface area of the melt was approximately 1864 cm2 or 2.01 ft2, this yields a power density of 12.1 kW/ft2. It is expected that most of the holes and pits would be treated using either a 3.5 or 4.5-m electrode spacing resulting in expected 28 or 17 kW/ft2 power densities, respectively. The pilot-scale test was run at a slightly lower power density than that which would be expected during large-scale processing. However, this does not affect the applicability of these test results to large-scale nor the ability of the large-scale process to treat the Holes and Pits effectively. Geosafe's large-scale ISV system incorporates a large power supply which provides operations personnel the ability to either decrease or increase power depending upon melting conditions, as well as, feeding or holding electrodes to obtain desired melt configurations. Ultimately, the size of the area to be treated will dictate which electrode separations are used, which may change from one location to the next.
A relatively linear melt rate was achieved after start-up. As expected, the melt rate slowed down later in the test. This is due to the fact that as the melt gets larger more energy is lost in heating surrounding soil. During a large-scale melt, typically the power to the melt would be increased to compensate for the increased heat loss to the surroundings. However, this test was performed at maximum output for the transformer so an increase in power could not be applied. The average melt temperatures during the test ranged between 1500 and 1600°C with the peak melt temperature reaching 1658°C. An average melt rate of 2.3 cm/hr was achieved during this test. This is consistent with typical melt rates achieved during large-scale operations.
The melting portion of the test proceeded very well, with no unusual affects resulting from the presence of the sealed containers, combustible organics, or debris. The surface of the melt exhibited only minor bubbling as soil gases were released into the plenum region. Combustion of organic vapors within the hood plenum area was observed. The minor bubbles rising to the surface of the melt is normal during ISV processing. Observations also indicated that the molten material exhibited a relatively high viscosity. A high viscosity melt is caused by high levels of glass-forming materials (SiO2, Al2O3, & Fe2O3). Latter in the melt, the molten surface cooled and formed a cold cap. Developments of cold caps later in the melts are typical.
Off-gas sampling was conducted at three different periods during the test. Off-gas sample results were used in evaluating the process destruction efficiency for organics and the retention of the radionuclide surrogates. Periods of the test were selected for off-gas sampling when the highest likelihood existed for melting into the sealed containers and thus exposing their contents.
The average off-gas flow rate during the test was 78.1 dscfm. A total of 22.4 L of condensate water was collected. This condensate results from the cooling of the off-gas stream which contains the vaporized soil moisture removed from the treatment volume. Mass balances performed on water from previous work show that all of the water vaporized in treatment volume is recovered as condensate or passed through the system in the saturated off-gas steam (Buelt and Bonner. 1989 and Timmerman and Peterson. 1990).
The test produced a block with the dimensions as presented in Table I. The mass of the vitrified block was calculated based upon conservative measurements, but was not actually weighed. It is expected that the vitrified product weighed between 727 and 909 kg (see Fig. 2).
Table I Vitrified Block Dimensions
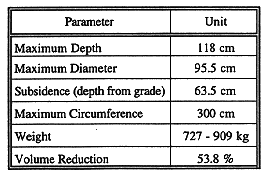
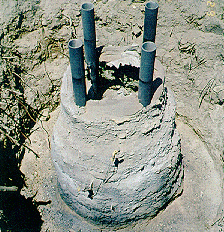
Fig. 2. Vitrified product prior to
removal.
The efficiency of the process is measured as a function of the amount of energy consumed during melting divided by the mass of the block produced. This test exhibited an estimated efficiency of 1.25 to 1.5 kWh/kg. Typical efficiencies for engineering-scale testing range from 1.0 to 1.5 kWh/kg. Large-scale efficiencies range from 0.8 to 1.0 kWh/kg. The higher efficiency at large-scale is due to a decrease in heat loss caused by a smaller melt surface area to volume ratio at large-scale. Full-scale processing of the BNL site using the ISV process should exhibit an excellent efficiency due to the low soil moisture present in the site soils.
Sampling of the soils surrounding the vitrified product and the vitrified product itself was performed once the test was completed. As can be seen in Table II, only minimal levels of acetone were detected in the surrounding soils and this concentration may be expected to decrease substantially during large-scale operations. Typical destruction efficiencies of all organics increases during large-scale operations due to prolonged exposure of contaminants to high temperature. Prolonged exposure to high temperatures is due to achieving a deeper melt depth at large-scale. During large-scale operations, the melt size can achieve a diameter of 9 m or more and depths of 6 m depending upon the size of the area to be melted (smaller melts similar to the BNL pits can also be performed). The larger melt size causes the organic contaminants to be exposed to high temperatures for much longer periods as they move through the dry zone around the melt. It should be noted that the gas-phase permeability in the dry zone does not change with varying size of melts or equipment, and thus, the dry zone always possesses a higher permeability than the non-thermally influenced surrounding soil because surrounding soil typically has some degree of water saturation. This longer exposure ensures more complete pyrolysis and destruction of organics.
TABLE II Pilot-Scale Treatability Test Analytical Results
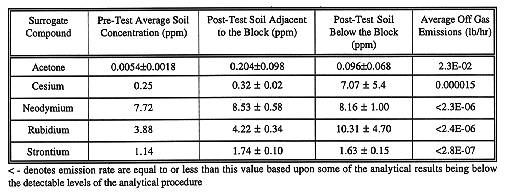
Based upon the amount of acetone detected in the off gas, and that detected as remaining in the soil, it was determined the substantially all of the acetone present in the test volume was either removed or destroyed.
The efficiency of the melting process in treating the organic contaminants is measured as the destruction efficiency (DE). Determination of the DE is as follows:
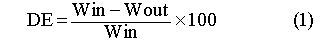
where, Win is the total mass of contaminant in the test chamber and Wout is the amount of contaminant measured in the off gas (40 CFR 1991). The destruction efficiency and the destruction and removal (via off-gas treatment) efficiency (DRE) have historically been based upon the regulations developed for incineration technology, where the quantity of contaminant present initially is known and the amount exiting the stack is measured and everything else is assumed to have been destroyed. This standard approach has been adopted since: ISV is a relatively new and innovative technology and no regulatory guidance has been issued for its operation, with the exception of treating PCBs for which Geosafe was recently granted a national TSCA permit, and the surrounding soils are routinely below or near the detection limit of the analytical procedure. This general approach of analyzing the efficiency of the ISV process has been used in all tests performed by Geosafe and reviewed routinely by EPA and other state and federal agencies.
The DEs for the process were calculated using the mean pre-test quantities of acetone placed in the containers. The DE calculations do not take into account the quantity of contaminant found in the surrounding soil, although this is small due to acetone being found at ppb levels in the surrounding soil. The calculated DE for acetone was determined to be 33%. Although this level is lower than expected, it has been demonstrated that during large-scale operations, improved organic destruction efficiencies would be obtained. Combining the DEs found during treatability testing with a 99.9 to 99.9999% removal efficiency for large-scale off-gas treatment, the total destruction and removal efficiency (DRE) would be approximately 99.93 to 99.99993%, for the acetone investigated in this testing. Geosafe's current large-scale off-gas treatment system is equipped with a thermal oxidizer as a final polishing step. The thermal oxidizer unit has demonstrated the ability to achieve >99.9% additional destruction of any organics.
Analyses performed on the post-test surrounding soil samples indicated the lack of significant increase in any of the surrogate contaminants present in the pit. Although all of the compounds were found present in the surrounding soils this is primarily due to the fact that all of the radionuclide surrogates were present in the pre-test soils (see Table II). In most cases, the concentrations of radionuclide surrogates found in the surrounding soil were within the statistical variability of the analytical methods. The results of the post-test surrounding soil samples are presented in Table II. For two of the compounds, there were slight increases in the surrounding soil concentrations that fell outside of the variance of the samples analyzed. In the case of strontium analyses performed on the soil adjacent to the block samples, the increase (from 1.145 ppm to 1.74 ppm) is not considered significant. The concentration of cesium in the samples taken below the block was also outside of the statistical confidence interval of the post-test surrounding soil compared to the pre-test levels (see Table II). There is a potential that a small amount of the 590 g of CsCl that was placed at the bottom of the test pit volatilized as the melt approached the container. Cesium chloride has a boiling point of 1290°C, and volatilization would occur at the outer edge of the melt. The three samples analyzed for cesium did have a large range of reported values (from 2.6 to 12 .4 ppm). These samples were taken directly below the block in a location immediately adjacent to the location of the cesium container, which may have accounted for residual material existing in the soil. The small amount of material remaining in the soil could have been captured by further overmelting, which is commonly done during large-scale operations. The average concentration of cesium (of 7.07 ppm) detected below the block accounts for less than 0.5 % of the concentration of cesium that was retained within the vitrified material. Rubidium was detected below the block at an average concentration of 10.31ppm; however, a confidence interval of almost ±5 ppm was determined due to the high variances of the sample population.
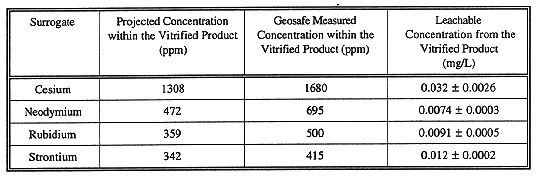
Four samples of the vitrified product were collected and extracted per 40 CFR 261, Appendix II (TCLP) procedure and analyzed for the radionuclide surrogates. The results of these analyses are presented in Table III. Only very small amounts of the radionuclide surrogates were leachable from the vitrified product ranging from 0.0074±0.0003 mg/L for Nd to 0.032 mg/L for Cs. To determine whether these levels are a concern, representatives of the State of New York Department of Environmental Conservation (NYDEC) were contacted. Based upon this conversation, there does not appear to be an approved clean-up standard for radionuclides in the soil at the BNL site. A policy has been proposed that would limit the dose rate in the soil to less than 10 mrem/yr, but has not yet been accepted.
TABLE III Post-Test Glass Analyses
Evaluation of the vitrified product was also performed by Fuhrmann et al. of BNL (Fuhrmann et al. 1996). Two different leach tests were performed consisting of an Accelerated Leach Test (ALT) and a Product Consistency Test (PCT). The report indicated that the release rates of the monolithic samples measured by the ALT were very low.
The PCT indicated that no detectable Cs or Rb were measured in the leachate. Nd and Sr were measured at 0.018 and 0.008 mg/L, respectively.
One sample of the glass material was also submitted for analysis to Chemex Labs, Inc for analysis of the total radionuclide surrogates contained within the vitrified product. Due to the vitrified product's resistance to digestion by standard methods, the vitrified product must be exposed to very aggressive acids to digest the material into a solution where it can then be analyzed (and this method is not always successful in completely digesting the material). For the rubidium and strontium analyses the samples were digested in a perchloric-nitric-hydrofluoric digestion followed by analysis using atomic adsorption spectroscopy. The neodymium and cesium were analyzed using neutron activation analysis. Table III presents the amount of surrogates retained within the block.
Sampling of the off gases generated during the test was performed for the radionuclide surrogates in accordance with EPA Method 5 in conjunction with trace metals measurement conducted per EPA Method 29. A sample of the off gas was withdrawn isokinetically from the off-gas line where it then passed through a heated quartz filter and a series of chilled impingers. All particulate matter was collected in the probe and filter, whereas gaseous emissions were collected in the dilute nitric acid/hydrogen peroxide solution impingers.
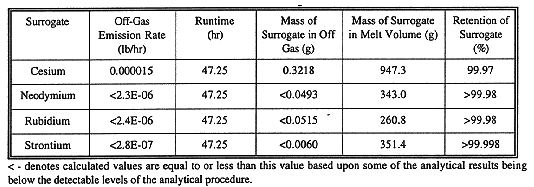
Table II presented the results of the radionuclide surrogate off-gas sampling. Three of the four radionuclide surrogates were below the detection limit when the particulate filter and the impinger solutions were analyzed. In these cases, the detection limit was used to calculate a less than" emission rate. Cesium was found in the off-gas stream at a concentration slightly above the detection limit. It should be noted that sampling of the off-gas stream occurred prior to any off-gas treatment. Cesium was initially present in the highest concentration in the test pit at nearly 300% of the other surrogates. Cesium is also one of the most volatile compounds of the radionuclide surrogates, thus it is not surprising that small quantities will volatilize and be collected in the off-gas system. The emission rate measured for cesium is very low and would easily be handled via conventional off-gas treatment currently being used by Geosafe (i.e., pre-filtering, wet scrubbing, and HEAP filtration). A recent large-scale test completed on a radioactive ORNL seepage pit demonstrated that only 0.0019% of the cesium present in the trench was released to the off gas (99.998% was retained in the melt). This was effectively removed via roughing and HEAP filtration (ORNL/ER-377).
The efficiency of retaining radionuclides surrogates within the ISV product is expressed as a percent retention in the vitrified product. It is defined as follows:
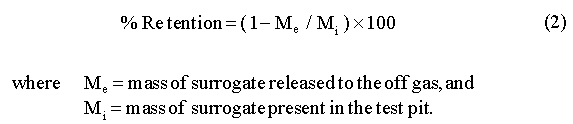
Table IV presents percent retention of the four radionuclide surrogates used during this test. Typical retention at large-scale for non-volatile radionuclides such as strontium or plutonium are in the range 99.99% to 99.999%. More volatile compounds such as cesium and cobalt are usually retained at slightly lower levels of 99% (Buelt, et al. 1989). Off-gas removal of the radionuclides enhances the total clean-up by removing an additional 99.99 to 99.999% of the very small portion that is volatilized to the off gas. The data gathered during the BNL pilot-scale test agrees with previously generated data. Data generated from the recently-performed large-scale radioactive demonstration at the Oak Ridge National Laboratory indicated that 99.9981% of the cesium present in the trench remained within the vitrified glass (ORNL/ER-377).
It should be noted that sampling of the off gases was not performed continuously, but rather during periods when emissions would have been at their peaks. As a result, the actual reported value for mass of surrogate released to the off-gas is most likely high. This would result in these values being conservative.
TABLE IV Retention of Radionuclide Surrogates in the Vitrified
Product
CONCLUSIONS
The ISV pilot-scale treatability test was successfully performed on actual BNL site soils containing simulated contaminants. Geosafe has evaluated the test data and arrived at the following conclusions.
REFERENCES