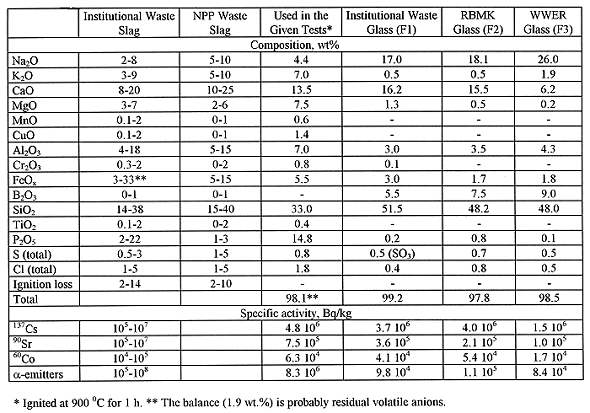
Table I Slag and Borosilicate Frit Compositions and Activities
T.N. Lashtchenova, F.A. Lifanov, and S.V. Stefanovsky
SIA "Radon", 7th Rostovskii per., 2/14, Moscow 119121 Russia
tel./fax 7-095-919-3194
ABSTRACT
Lab-scale experiments with actual Radon incinerator radioactive ash (slag) were conducted. Granulometric, chemical, and phase compositions were determined. Major crystalline phases were established to be calcium phosphates and silicophosphates. Borosilicate frit and commercially available sodium silicate were proposed as glass forming additives. Effect of temperature on melts viscosity and resistivity was studied. It has been shown that compositions with slag (ash residue) content of 50 wt% and higher have good chemical durability. 137Cs leach rate from such materials is ~10-6 g/(cm2 day) and lower. Waste products has strong physical integrity. Waste volume reduction factor ranges between 4 and 7. Material structure by infra-red spectroscopy and EPR was also studied.
INTRODUCTION
The basic method of burnable waste volume reduction is incineration (1). Incineration reduces waste volume by factors of 50 to 100. As a rule, solid burnable waste contains both burnable organic and non-combustible inorganic constituents. Organic constituent decomposes, yielding gaseous phase and ash. Off-gas is subjected to wet and dry cleaning with formation of condensate and purified gas. Inorganic constituent is also transformed. Ash and other inorganic species form slag (ash residue). This slag is dusting and easy water leachable material not suitable for direct disposal. Slag can be transformed in stable monolithic form by cementation or bituminization (1). However, these methods can not ensure safe isolation of radionuclides from biosphere due to insufficient chemical durability and low mechanical strength of composites. Moreover, ash-bitumen composites are burnable. Another disadvantage of ash cementation and bituminization is very low ash volume reduction factor. Volume of ash-cement block is often more than volume of initial ash by 10-20%.
By these reasons, new ash immobilization methods are under development now. For the time being, two principal trends such as melting/vitrification and ceramization are considered. Melting route provides for either direct flux free slag melting in microwave (2) or inductive ("cold crucible") (3) melters, or slag vitrification with flux (4-7). Flux free slag melting requires high temperatures (1400-1550°C) because of high melting point of inorganic constituents, including calcium phosphates and silicophosphates. Flux application decreases process temperature down to 1100-1300C. Boron-containing additives (3-5), basalt (6), low fusible another radioactive waste (3,4,7) are used as flux. Resulting products have high chemical durability, good mechanical strength, and small final volume. These have also very good radiation stability (8).
In Russia, great ash volumes after solid and liquid burnable radioactive wastes treatment are also produced at Nuclear Power Plants (NPP) and Research Centers. At the present time this ash is cemented or charged into barrels and buried directly without additional treatment. Such disposal methods do not provide sufficient safety level for long-term storage. So, incorporation of incinerator ash (slag) in glass or glass crystalline materials is strongly recommended. In the present work we selected suitable fluxing (glass forming) additives and determined principal properties of initial and vitrified slags. Slag obtained at Radon chamber incinerator "Fakel" (9) was taken for investigation. This slag composition (Table I) is typical for Russian NPP and institutional wastes as wastes to be incinerated are approximately the same. Usual waste constituents are cellulose, rubber, plastics, biomaterials as well as glass, ceramic, concrete breakage, and metal.
Table I Slag and Borosilicate Frit Compositions and Activities

Slag composition is appreciably varied from batch to batch. Two samples of slag from Radon incinerator were examined. Range of particle dimensions was determined as follows. About 50 kg of slag was discharged and classified. Large metal reinforcement, ceramic and concrete pieces, bones, etc., with dimension 100 mm and more were removed. Then, fractions 50-100 mm, and 10-100 nun were separated and their weight fractions were determined. Residue with particle size less than 10 mm was sieved using standard sieves.
In order to prepare the slag for lab-scale experiments, slag portions (2 kg each) after removal of large pieces (>50 mm) was ball-milled for 30 rain.
Before chemical analysis of the slag, ignition loss was measured. The slag was heated to 900°C and kept at this temperature for 1 h followed by cooling down to room temperature. Ignition loss corresponding approximately to organic compounds content, was calculated as the difference between initial and final weights of the slag.
The slag after heat treatment was re-heated up to 1000°C and kept at
this temperature for 3 h to remove residual volatile anions (mainly carbonates
and hydroxyls). Prepared slag was analyzed by emission spectral analysis
(spectrometer ISP-30, Russia) and atomic absorption spectroscopy
(spectrophotometer S-115, Russia). Results are given in Table I. X-ray
diffraction analysis (XRD) was performed using DRON-4 diffractometer (Russia),
Cu K![]() radiation. Specific activity of samples (Table I) was measured using SI-8B
counter equipped with PSO 2/4 converter (
radiation. Specific activity of samples (Table I) was measured using SI-8B
counter equipped with PSO 2/4 converter (![]() -
- -emitters), and Tl-activated ZnS
detector with FEU-19 multiplicator (
-emitters), and Tl-activated ZnS
detector with FEU-19 multiplicator ( -emitters). Radionuclide
composition was determined using
-emitters). Radionuclide
composition was determined using
 -spectrometer based on
LP-4900 "Nokia" (Finland) analyzer and equipped with semiconductive
detector DGDK-80 by comparison with OSPI standard sources.
-spectrometer based on
LP-4900 "Nokia" (Finland) analyzer and equipped with semiconductive
detector DGDK-80 by comparison with OSPI standard sources.
Three borosilicate glass frits (Table I) contained high sodium intermediate-level institutional (Moscow Waste Reprocessing Station - MWRS), RBMK and WWER wastes, and sodium silicate Na20 nSiO2 (2.5<n < 3) were examined as glass forming additives (4,8). Slag and flux were mixed in alumina crucibles and placed into resistive furnace. Batches containing slag and borosilicate frit or dry sodium silicate were heated to melting temperature for 2 h and kept for 1 h. Batches containing slag and "soluble glass" (sodium silicate water solution) were heated to 150°C and kept until all water was removed followed by heating to melting temperature and holding. Then furnace was turned off and crucibles were cooled into the furnace. Some samples were prepared by melt quenching onto cold steel plate.
Melt viscosity and resistivity was measured using system based on GOI vibrational viscosimeter (State Institute of Glass design (10)) modified in Radon.
Leach rate of radionuclides was measured by IAEA (MCC-1) technique (11). Compressive strength was determined using standard laboratory press. Waste volume reduction factor was also calculated.
In order to study some features of glass structure, location, and valency
state of ions, infra-red (IR) spectroscopy (Fourier-spectrometer Perkin-Elmer
PE-1700, pressing of glass powder in KBr pellets) and electron paramagnetic
resonance (EPR) were used. EPR spectra of initial and
 -irradiated to absorption
dose 100 kGy samples of vitrified slags were recorded using Bruker ESP-300
spectrometer operated at X-band at -196 and 20°C.
-irradiated to absorption
dose 100 kGy samples of vitrified slags were recorded using Bruker ESP-300
spectrometer operated at X-band at -196 and 20°C.
RESULTS
Slag Characterization
Largest fraction (>100 mm in size) in source slag accounts for more than 30 wt%. This fraction can not be charged into the melter or milled. Fraction with particle size ranged between 10 and 100 mm can not also be charged into the melter, but it can be ball-milled in order to be added to feed composition. Its weight fraction is approximately 10%. More fine-grained slag fractions may be used directly for batch preparation to be fed into the melter.
As seen from chemical analysis data (Table I), sum of silicon, phosphorus, aluminum, and calcium oxides accounts for more than 50 wt% of total. These oxides are able to form glasses or glass crystalline materials especially in combination with sodium, potassium, magnesium, and iron oxides being also presented in the slag. However, high content of high-fusible silica and alumina results in very high slag melting point. In order to lower melting temperature fluxing agents must be added to the slag.
It should be noted that sulfates and chlorides in the slag also occurred. Chlorides form probably by reaction between metal oxides and chlorine or hydrogen .chloride formed by PVC decomposition. Sulfate and chloride formation can result in phase separation at melting (12).
Hydroxylapatite and calcium silicophosphates such as silicocarnotite Ca5(SiO4)(PO4)2 and nagelschmidtite Ca7(SiO4)2(PO4)2 are found to be as major mineral phases in the slag. Hydroxylapatite is predominant crystalline phase in the source slag while silicophosphates occurred in re-heated, melted and vitrified slags. Their formation is obviously caused by solid phase reactions between hydroxylapatite and silica at high temperatures, for example,
or
Ca5(PO4)3(OH) + SiO2 = Ca5(PO4)2(SiO4) + HPO3 (1)
Cas5(PO4)3(OH) + 2 SiO2 = Ca7(PO4)2(SiO4)2 + HPO3 (2)
Significant amount of amorphous phase was also found. Alkali aluminosilicates (plagioclases, and nepheline), iron and iron silicide was presented as minor phases. Iron silicide possibly formed by reaction between iron and silica under reducing conditions. Slag heated at 1000°C for 3 h did not reach constant weight possibly due to vaporization of metaphosphoric acid as follows from reactions 1) and 2), and volatilization of other components.
Radiochemical analysis of the slag indicated inhomogeneous partitioning of
radionuclides in the bulk and significant variation of each radionuclide content
in the different parts of each sample. Some samples were enriched with
 -emitters.
-emitters.
Development of Slag-Containing Borosilicate Glasses
Vitrification of liquid high-sodium low- and intermediate-level waste (LILW) with borosilicate glass forming additives yields sodium borosilicate glass as wasteform. Glasses for immobilization of institutional LILW, NPP with RBMK and WWER wastes were developed at SIA "Radon" (13). Glass compositions are given in Table I. These glasses contain either up to 30-35 wt% institutional or RBMK wastes, or up to 40-45 wt% WWER waste. They have relatively low viscosity in order to dissolve most of the slag components, and resistivity suitable for electric melting (Fig. 1 and 2).
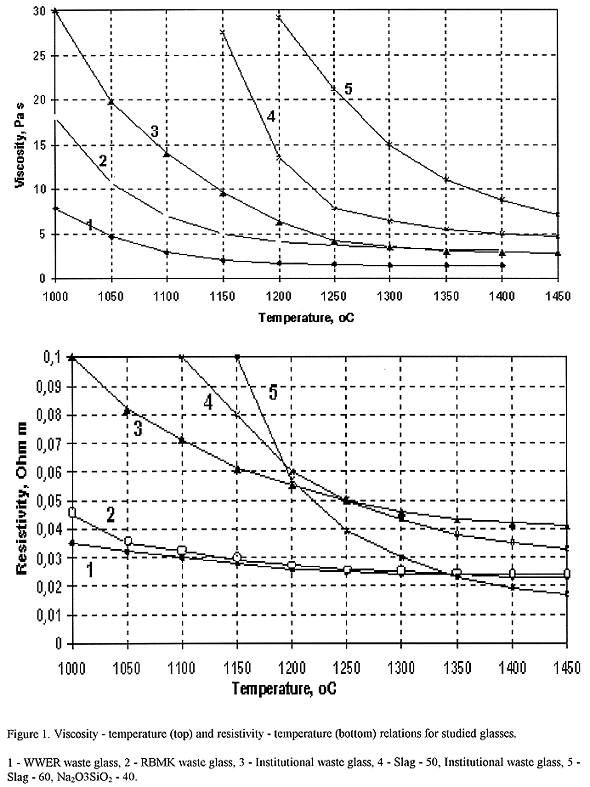
Fig. 1. Viscosity - temperature (top)
and resistivity - temperature (bottom) relations for studied glasses. 1- WWER
waste glass, 2 - RBMK waste glass, 3 - institutional waste glass, 4 - slag - 50,
institutional waste glass, 5 - slag - 60, Na2O3SiO2
- 40.
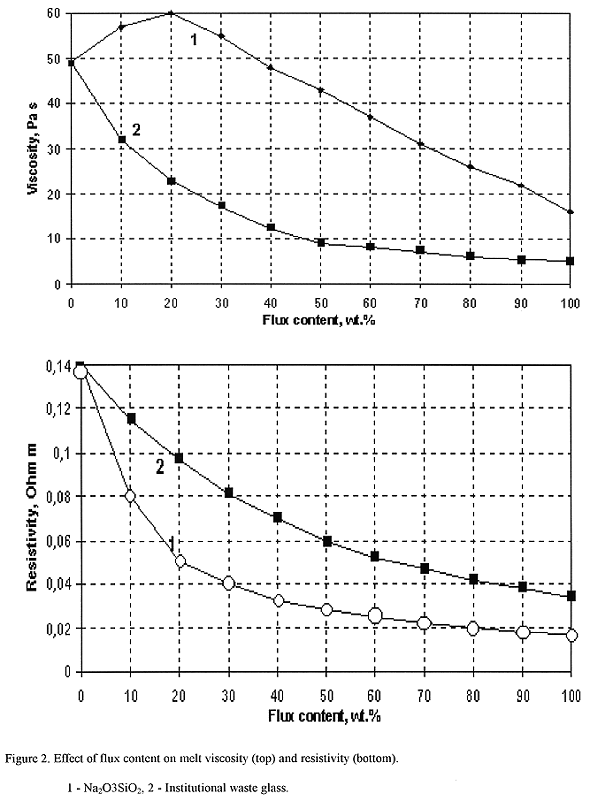
Fig. 2. Effect of flux content on
melt viscosity (top) and resistivity (bottom). 1 - Na2O3SiO2,
2 - institutional waste glass.
Melts containing 0...50 wt% slag oxides and 50... 100 wt% borosilicate frit are suitable for electric melting at 1200°C. Measured viscosity and resistivity are characteristics of homogeneous melts. Real process temperature must be elevated by 50-150°C. In order to increase slag oxides content in glasses, process temperature must be still raised. Formation of homogeneous melt containing 50 wt% and 60 wt% slag oxides takes place at 1250°C and 1350°C, respectively. Effect of temperature on borosilicate melts viscosity and resistivity are also shown on Fig. 1.
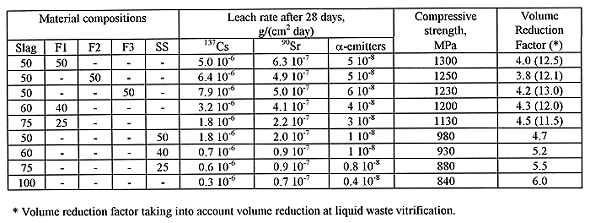
The main properties of products are given in Table II. Leach rate of 137 Cs after 28 days of examination is (1...4) 10-6 g/(cm2 day), compressive strength ranges between 0.8 and 1.5 GPa.
Slag volume reduction factor is approximately 4, but total waste volume reduction factor, taking into account the presence of liquid waste oxides in glass, reaches 10.
Table II Properties of the Vitrified Slags
Development of Slag-Containing Boron Free Silicate Glasses and Glass Crystalline Mmaterials
Various sodium silicates Na20 nSiO2 (2 < n < 4) may be applied as fluxing and glass forming agents (9). Commercially available "soluble glass" - sodium silicate with composition being close to Na2O (2.5...3)SiO2 is preferable for industrial-scale process. Impregnation of the slag with "liquid glass" excludes dusting and ensures good feed homogeneity.
Melting temperatures of slag-sodium trisilicate mixtures are higher compared to slag-borosilicate frit mixtures with the same slag oxides content by 100-200°C.
Effect of slag to Na2O3SiO2 ratio on melt viscosity and resistivity are shown on Fig. 2. Maximum observed on viscosity-composition relation is connected to features of melt structure. Stucturization of slag-containing inhomogeneous melts was observed elsewhere (14).
As seen from Figs. 1 and 2, viscosity of slag-containing melts is high and significant melt overhearing at melting process can be required. It should be noted that molten slag viscosity is strongly depended on its composition and may be varied significantly.
Comparison of viscosity-temperature relations indicates that slag-sodium silicate melt is "shorter" than slag-borosilicate melt (Fig. 1). Slag-sodium silicate melt is more conductive than slag-borosilicate melt at temperatures 1200°C and higher.
Slag-sodium silicate materials are very chemically durable. Leach rate of 137 Cs from product containing 60 wt% slag oxides and 40 wt% sodium trisilicate after 28 days of exposure in distilled water was 8 10-7 g/(cm2 day) (see Table II). Cesium leach rate from materials with higher slag content, including melted flux free slag, is still lower (~108 g/(cm2 day)) but melting temperatures for such materials are too high (1500°C and higher).
Compressive strength and waste volume reduction factor measured for material containing 60 wt% slag oxides content are given in Table II.
Mineral Composition and Structure of Materials
Materials prepared by smelting of the slag with borosilicate frit were predominantly amorphous. Their appearance was typical for glass. However, small amount of crystalline phases was revealed. Only the same crystalline phases as in the slag pre-heated to 900°C were observed. Their content was estimated to be as many as 5-10%.
Slag-sodium silicate materials containing up to 80 wt% slag prepared by quenching were also predominantly amorphous and contained small amount of crystalline phases being approximately the same as in the slag pre-heated to 900°C. However, new crystalline phases, leucite KAISi2O6 and kaliophilite KAISiO4 were determine in slowly cooled samples. Obviously, these phases, as well as major fraction of nepheline, were crystallized from the melt. Total crystalline phases content in slowly cooled samples, containing 50-80 wt% slag oxides, was estimated to be approximately 60-80%. Total crystalline phases content in the melted flux free slag reached 85-90%. Major phases were nepheline K0.33Na0.67AISiO4, and silicophosphates. Moreover, numerous minor phases, including leucite and kaliophilite, were observed.
IR spectra of materials are shown on Fig. 3. IR spectra of slag-borosilicate products, containing 50-75 wt% slag oxides, are similar. In the interval of 2000-380 cm-1 they consist of bands located at 1350-1500, 850-1200, 700750, and 400-550 cm-1. IR spectra of slag-sodium silicate materials, as well as melted slag, contain strong broad bands located at 850-1200, 650-750, and 400-600 cm-1. The latter band is doublet consisting of two narrower bands centered at 570 and 460 cm-1. The increase of slag content in materials results in symmetrization of the band located at 8501200 cm-1 with maximum at 1030-1035 cm-1, and the gain of the band centered at 570 cm-1.
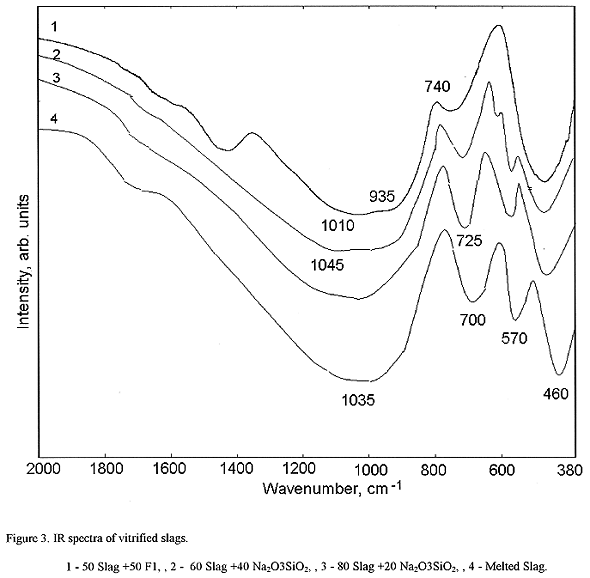
Fig. 3. IR slag +50 F1, 2 - 60 slag
+40 Na2O3SiO2, 3 - 80 slag +20 Na2O3SiO2,
4 - melted slag.
The band at 1350-1500 cm-1 is present in IR spectra of
slag-borosilicate materials and absent in spectrum of slag-sodium silicate
materials. Therefore, this band relates to vibrations within boron-oxygen
groups. It is attributed to vas vibrations of B-O bonds in boron-oxygen
triangles (15). The rest of bands may be assigned to vibrations of
silicon-oxygen groups (vibrations of BO4 tetraheclra can contribute
to the band located at 850- 1200 cm-1). The band located at 850-1200
cm-1 is superposition of bands related to vas Si-O-Si
(major contribution), bonding SiO4 tetrahedra in glass network, and
vas Si-O- (minor contribution). The latter band centered
at 930-940 cm-1 iswell-resolved in IR spectrum of slag-borosilicate
glass. The decrease of SiO4 tetrahedra with non-bridging oxygen ions
is responsible for symmetrization of the band located at 850-1200 cm-1.
The band located at 700-750 cm-1 may be attributed to vs Si-O-Si
vibrations. Absorption bands centered at 570 and 460 cm-1 are
components of  as
and
as
and  s
vibrations of SiO4 tetrahedra. Splitting of single line located at
400-550 cm-1 is due to symmetry depression of SiO4
tetrahedra caused by partial devitrification. It is in good agreement with fact
that crystalline phases fraction rises with the increase of slag content in
material.
s
vibrations of SiO4 tetrahedra. Splitting of single line located at
400-550 cm-1 is due to symmetry depression of SiO4
tetrahedra caused by partial devitrification. It is in good agreement with fact
that crystalline phases fraction rises with the increase of slag content in
material.
As follows from IR spectra, slag-containing borosilicate glass network is built from SiO4 tetrahedra and complex borate groups containing both three- and four-fold coordinated boron. The "connectedness" degree of this network is quite high. Vitreous phase of slag-sodium silicate materials is also built from chains formed by SiO4 tetrahedra (as well as AIO4, and, possibly, PO4 tetrahedra), and network "connectedness" degree becomes higher with the increase of slag content. Simultaneously, total crystalline phases content increases.
EPR spectra of slag-borosilicate and slag-sodium silicate materials contain
responses with g-factors located at ~9.5, 4.3 and 2.0 due to Fe (Ill) impurity
(Table III). Spectrum of the melted slag is complex line. It has been shown by
computer analysis and simulation that this line is formed by two overlapping
spectra. One of them is broad line due to Fe3+ ions (line width
![]() H
H 50 mT, g
50 mT, g 2.0) and the
second one is narrower line with axial symmetry and g|| =2.37, and g|
=2.08 due to unresolved hyperfine structure of Cu2+ ions
(electronic configuration 3d9, nuclear spin I=3/2 for both
63Cu and 65Cu). Only weak responses due to Cu2+
-ions in EPR spectra of materials with 75% and 60% slag content were observed.
Moreover, very weak six-component signal due to Mn2+ ions
(interaction of unpaired electron with 55Mn nucleus, 1=5/2) was
observed in these spectra (Table III).
2.0) and the
second one is narrower line with axial symmetry and g|| =2.37, and g|
=2.08 due to unresolved hyperfine structure of Cu2+ ions
(electronic configuration 3d9, nuclear spin I=3/2 for both
63Cu and 65Cu). Only weak responses due to Cu2+
-ions in EPR spectra of materials with 75% and 60% slag content were observed.
Moreover, very weak six-component signal due to Mn2+ ions
(interaction of unpaired electron with 55Mn nucleus, 1=5/2) was
observed in these spectra (Table III).
Table III Parameters of EPR Responses
Spectra of 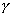 -irradiated
slag-borosilicate and slag-sodium silicate glassy materials contain narrow
resonances located near ge (Table III). Narrow resonance in EPR
spectrum of slag-borosilicate glass is due to overlapping of boron-oxygen hole
center (BOHC) with four-fold coordinated boron (four lines caused interaction of
unpaired electron with 11B nucleus, I=3/2 (16)), and silicon-oxygen
hole center (SOHC). The latter may be identified as HC2 -center
(trapped hole at SiO4 tetrahedron with two non-bridging oxygen ions
(17)).
-irradiated
slag-borosilicate and slag-sodium silicate glassy materials contain narrow
resonances located near ge (Table III). Narrow resonance in EPR
spectrum of slag-borosilicate glass is due to overlapping of boron-oxygen hole
center (BOHC) with four-fold coordinated boron (four lines caused interaction of
unpaired electron with 11B nucleus, I=3/2 (16)), and silicon-oxygen
hole center (SOHC). The latter may be identified as HC2 -center
(trapped hole at SiO4 tetrahedron with two non-bridging oxygen ions
(17)).
Narrow line in EPR spectrum of irradiated silicate-based material with 75% slag content is also present. This complex line consists of two overlapping resonances due to HC1 (trapped hole at SiO4 tetrahedron with one non-bridging oxygen ion (17)) and HC2 - centers. Their g values are usual for these centers. Response of trapped electron center (electron located near alkali or alkali earth ion (17)) is also observed (Table III).
DISCUSSION
Granulometric data show this factor is considered to be one of the most important for determination of principal slag treatment process flowsheet and melter design. The presence of largest fraction (metal reinforcement, large pieces of glass, ceramics, bones) is the most troublesome. Occurrence of large fraction, including metal, does not give rise essential problems at pot vitrification, especially as far as concerned systems with indirect heating (resistive furnaces and intermediate frequency (1-100 kHz) inductive melters with heating of metal crucible walls). In this case large lumps can finally be either dissolved in the melt (for long time thus decreasing process capacity), or fixed into the melt as unaffected or partially altered.
Application of high frequency (300-10000 kHz) inductive melting with a cold crucible with periodic mode of operation allows to melt inorganic constituent of the slag but metal constituent creates a problem. Molten metal collects at the bottom part of the cold crucible and, as a result, certain zone of the cold crucible is excluded from the process, and crucible displacement with respect to inductor. (The same operation should be done if melting process is performed by rising level method.) However, normal operation disruption at ingress of metal reinforcement or other large lumps is most probably.
Application of the cold crucible with semi-continuous mode of operation and bottom melt discharge processes the slag without sizing. In this process molten metal and other large lumps are collected at the crucible bottom, then, the bottom is hinged out, and contents are discharged into container. Large lumps are flooded with molten slag producing solid block. The same container will be used for final disposal.
The use of Joule heated ceramic melter or the cold crucible with continuous mode of operation for slag vitrification requires pre-sizing to separate metal constituent and large lumps. These can break electrodes or connect their to chassis earth in Joule heated ceramic melter or decrease cold crucible operating volume. Unlike the cold crucible equipped with dumping bottom, a given metal can not be removed that results in process disruption, and crucible degradation and process failure.
Thus, application of the "cold crucible" with periodical or semi-continuous mode of operation is expedient for treatment of the slag with significantly variable composition or slag without additional treatment (unclassified). Application of resistive furnaces is the less advantageous due to very low capacity. Vitrification of incinerator ash of pre-sorted waste or pre-sorted slag is available in the "cold crucible" at continuous mode of operation or in Joule heated ceramic melter, but the latter is the less advantageous because of its very high sensitivity to process variables and melt composition variations.
The slag is high-fusible material. Silica, alumina, and
 -whitlokite have the
highest melting points (1713, 2050 and 1810°C, respectively). Whitlokite,
silicocarnotite and nagelschmidtite are almost insoluble in silicate melts as it
has been shown elsewhere (18) and by ourselves as well (19). These are present
in materials as individual phases. Nevertheless, as seen from Table II, chemical
durability of slag-containing wasteforms remains high and presence of
silicophosphate phases in materials does not impact on cesium leach rate. Leach
rate of 90Sr can be even reduced, compared to homogeneous glass, due
to isomorphous substitution Sr2+ for Ca2+ in these
minerals.
-whitlokite have the
highest melting points (1713, 2050 and 1810°C, respectively). Whitlokite,
silicocarnotite and nagelschmidtite are almost insoluble in silicate melts as it
has been shown elsewhere (18) and by ourselves as well (19). These are present
in materials as individual phases. Nevertheless, as seen from Table II, chemical
durability of slag-containing wasteforms remains high and presence of
silicophosphate phases in materials does not impact on cesium leach rate. Leach
rate of 90Sr can be even reduced, compared to homogeneous glass, due
to isomorphous substitution Sr2+ for Ca2+ in these
minerals.
Addition of borosilicate frit to the slag results in the decrease of melt viscosity and resistivity in the whole interval compositional variation - from the slag to borosilicate glass. This is due to the increase of alkali oxides content in melts at level alkali earth content, mainly CaO, and insignificant increase of silica content. Viscosity temperature and resistivity - temperature relations are conventional for melts with fixed compositions (Fig. 1).
Effect of slag-sodium silicate melts composition on melt viscosity is more complex compared to slag-borosilicate melts. At 1300°C minor dope of sodium trisilicate to slag (up to 20 wt%) results in the increase of melt viscosity. Within this compositional range silica content in the melt increases quicker than flux (alkali .oxides). Viscosity decreases if sodium trisilicate content becomes higher than 20 wt%, and melt viscosity tends to pure sodium trisilicate viscosity (~16 Pa s (20)) due to permanent increase of alkali oxides content in the melt.
It should be noted, that melt resistivity values at slag content up to 100 wt% (molten slag) are suitable for electric melting, including the "cold crucible" melting. The limitation exists for viscosity. Melts with high slag content are too viscous (more than 10 Pa s) and very difficult for homogenization, and consequently, process capacity decreases. Application of the "cold crucible" melting, being distinguished by vigorous melt agitation with eddy currents in the crucible, can increase slag content in the final wasteform up to 100 wt%. Chemical durability of these products is suitable for long-term waste storage.
It has been shown from investigation of materials structure that
borosilicate materials contain mainly vitreous phase (crystalline phases content
is as many as 5-10 %). The structure of vitreous phase is very similar to
structure of initial borosilicate glass, which is Radon intermediate-level waste
glass whose structure has been well-studied (21). The increase of slag content
in slag-sodium silicate materials results in the increase of crystalline phases
content in materials. By this, a part of network modifiers enters crystalline
phases, for example sodium and potassium enter nepheline, potassium also enters
leucite and kaliophilite, calcium and magnesium enter silicocarnotite and
nagelschmidtite, aluminum enters aluminosilicates, etc., and vitreous phase is
enriched with silica yielding the increase of network "connectedhess"
degree as follows from IR spectra and EPR spectra of
 -irradiated materials.
-irradiated materials.
EPR responses with g-values ~9.5, 4.3 and 2.0 are usually assigned to Fe3+
ions (22). First two of responses were attributed to four-fold coordinated Fe3+
ions, and the latter is due to six-fold coordinated Fe3+ ions.
Intensity of the responses with g 9.5 and g
9.5 and g 4.3 decreases as far as slag content in slag-sodium
silicate materials increases. Intensity of the response with g
4.3 decreases as far as slag content in slag-sodium
silicate materials increases. Intensity of the response with g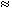 2.0 remains approximately the same. It indicates that
silicate materials with high slag content contain significant fraction of Fe
ions as Fe2+ whose EPR response is not observable at neither room
nor liquid nitrogen temperatures.
2.0 remains approximately the same. It indicates that
silicate materials with high slag content contain significant fraction of Fe
ions as Fe2+ whose EPR response is not observable at neither room
nor liquid nitrogen temperatures.
Spectral parameters of Cu2+ ion are typical for this ion in glass (23) while hyperfine structure constant value for Mn2+ (AMn = 10.5 mT) indicates that Mn-O bond in the first coordination sphere of Mn ion is predominantly ionic rather than covalent. This is probably due to Mn2+ ions enter mineral structure substituting for K+ or Ca2+ ions (24).
ECONOMICAL ASPECTS
In order to compare the incinerator ash vitrification process with cementation process from economical point of view, we should consider both of the processes in application to the same waste incineration plant. We used basic characteristics of the Radon incinerator <<Fakel>> (9). These are as follows, solid waste capacity - 80-100 kg/h, liquid waste capacity - 15-20 l/h, waste volume reduction factor - up to 100. Economic efficiency was determined by evaluation of specific expenses and prevented environmental damage.
At the same ash productivity, process area, facility cost, and salary the vitrification process requires higher electric consumption (by factor of ~3), but, due to higher product quality, environmental damage is significantly less compared to cementation method. Total specific expenses for the ash vitrification process are approximately the same as those for the cementation process, but taking into account prevented environmental damage and expenses for transportation of solidified product from processing site to regional repository, total economic efficiency of the vitrification process exceeds that for cementation process by factor of 2-3. For example, for SIA <<Radom>> an annual economic efficiency of the vitrification method with following transportation of the vitrified product to regional repository is higher by factor of 3.5 than without product transportation.
CONCLUSION
Slag (incinerator ash residue), containing both network formers and network modifiers, is high fusible and requires temperatures up to 1500-1600°C to be melted. It can be vitrified with borosilicate or sodium silicate flux, and borosilicate glass and silicate-based glass crystalline materials are formed. Melt viscosity and resistivity values permit electric melting of materials including the cold crucible melting. A necessity of slag pre-sorting is determined in each case depended on melter design, mode of operation and required process conditions. The structure of borosilicate materials is similar to the structure of borosilicate glass for immobilization of intermediate-level Radon waste. Leach rate of 137Cs is 10-7 - 10-7 g/(cm2 day). Leach rates of 90Sr and actinides are lower than 137Cs by 1-3 orders of magnitude. Waste volume reduction factors range between 4 and 7.
REFERENCES