M. Eric Schlienger, Joanna M. Buckentin and Brian K.
Damkroger
Sandia National Laboratories
Albuquerque, New Mexico
ABSTRACT
Nuclear operations have resulted in the accumulation of large quantities of contaminated metallic waste which are stored at various DOE, DOD, and commercial sites under the control of DOE and the Nuclear Regulatory Commission (NRC). This waste will accumulate at an increasing rate as commercial nuclear reactors built in the 1950s reach the end of their projected lives, as existing nuclear powered ships become obsolete or unneeded, and as various weapons plants and fuel processing facilities, such as the gaseous diffusion plants, are dismantled, repaired, or modernized. For example, recent estimates of available Radioactive Scrap Metal (RSM) in the DOE Nuclear Weapons Complex have suggested that as much as 700,000 tons of contaminated 304L stainless steel exist in the gaseous diffusion plants alone. Other high-value metals available in the DOE complex include copper, nickel, and zirconium. Melt processing for the decontamination of radioactive scrap metal has been the subject of much research. A major driving force for this research has been the possibility of reapplication of RSM, which is often very high-grade material containing large quantities of strategic elements. To date, several different single and multi-step melting processes have been proposed and evaluated for use as decontamination or recycling strategies. Each process offers a unique combination of strengths and weaknesses, and ultimately, no single melt processing scheme is optimum for all applications since processes must be evaluated based on the characteristics of the input feed stream and the desired output. This paper describes various melt decontamination processes and briefly reviews their application in developmental studies, full scale technical demonstrations, and industrial operations.
INTRODUCTION
The life expectancy of a commercial nuclear power plant is about 40 years. Those plants which began operation in the 1950s will be reaching the ends of their projected lives in the 1990s and additional plants are scheduled for shutdown through the year 2020. Each of these plants will produce 40,000 to 50,000 tons of metal waste when dismantled. Contaminated metal waste represents a considerable storage volume as well as a significant cost since it must be maintained and monitored indefinitely in secure storage. Alternatively, this material may be disposed of by burial, with current commercial costs being in the range of $450.00 per cubic foot.1 The resulting financial and environmental burden will continue to be a problem as quantities of contaminated waste increase while the space available for disposal continues to decrease. The high cost of either disposal or storage requires that the volume of material be minimized. Melting and casting into ingots of convenient shape reduces the volume of the material. However, if sufficient decontamination were achieved during melting, controlled reuse of the metal may be possible. Such reuse is an attractive option for many reasons, both economic and environmental. Americas stockpile of radioactive scrap metal represents a large domestic resource for elements such as nickel, chromium, and manganese. (1) Unless decontaminated and recycled, this resource is lost. Published results show that melt refining can be an effective technique for decontamination, as well as volume reduction, when the proper slags, refractories, and melting techniques are used.
Radioactively contaminated scrap metal arises from several different types of operation. The material composition and the type, level, and chemical form of contaminant present will vary depending on its source. In addition to requiring different processing schemes and/or parameters for optimum decontamination these differences will also affect environmental, safety, and health concerns for material handling and process operation. Virtually all facilities which have been sites of nuclear activity will, at some point, become sources for radioactive scrap metal. Commercial nuclear power plants are by no means the only source of radioactive scrap metal. Each step in uranium beneficiation processes results in uranium contaminated equipment. The utilization of this uranium for the production of power or weapons results in equipment contaminated not only with uranium, but with fission and activation products. Fuel handling, storage, and reprocessing result in yet more contaminated metal.
Dyer (2) surveyed the radionuclides found in commercial nuclear power reactors in the United States and found that the radionuclide types and amounts differ according to reactor type. Radionuclides resulting from activation of iron, cobalt, and zinc were shown to be present at similar levels in pressurized water reactors and boiling water reactors, unlike the activation nuclides for nickel and tin. The nuclides resulting from the activation of nickel, 58Co and 63Ni, were found to be present at a higher level in pressurized water reactors. The tin activation nuclide, 125Sb, was present at higher levels in boiling water reactors due to the dissolution of tin from the condenser tubes. Isotopes of radiocesium, 134Cs and 137Cs were shown to be two orders of magnitude higher in pressurized water reactors than in boiling water reactors, while isotopes of radiostrontium, 89Sr and 90Sr were found to be present at the same level in the two reactor types. Analysis of pressurized water reactor coolant indicated that 90% of the radionuclide activities were radiocesium fission products and 10% were iron and cobalt activation products (55Fe and 60Co). A similar analysis of boiling water reactor coolant revealed about 9% of the activities were from 125Sb, with the other 9% represented by 55Fe and 60Co. A survey of reactor parts showed that the radionuclides which accounted for 1% or more of the total activity were 54Mn, 55Fe, 60Co, 65Zn, 125Sb, 134Cs, and 137Cs.
Melt Decontamination
Melt decontamination of radioactive scrap metal has been widely studied and has several inherent advantages. These processes operate above the melting point of the scrap metal so that decontamination reactions take place either between two liquid phases or between a liquid phase and a gas phase, making the reaction kinetics involved very rapid. The high temperature at which these processes operate also aids their kinetic efficiency. When the radionuclide to be removed has a higher vapor pressure than its host metal, melting technologies can take place under vacuum in order to facilitate the removal of these species. Some melting technologies support the use of slags or fluxes which interact thermochemically and electrochemically with the liquid metal to specifically capture radionuclides. When properly formulated, these slags act as a low volume, stable containment for radioactive species. The basic operations of several processes are described below, as are design requirements necessary for each process to be used for radioactive materials.
The technologies which have been investigated for the melt decontamination of radioactive scrap metals include electric arc melting, air induction melting, vacuum induction melting, and electroslag remelting. In the following sections, each technique is described and the inherent advantages or disadvantages regarding the use of each as a decontamination strategy are briefly discussed.
Electric Arc Melting
In electric arc melting, the charge is heated and melted due to the electricity passed between an electrode and the charge (in direct current melting) or indirectly between three electrodes (in three phase alternating current melting). Electric arc furnaces consist of a refractory lined hearth and a water cooled roof section, with holes to allow the electrodes to be lowered into place. The roof section may be lifted or swung away to permit feedstock to be loaded into the furnace. The roof section is then replaced, the electrodes are lowered and power is applied to melt the charge. After melting, the furnace is tilted, slag is tapped, and the molten metal is poured into a ladle. This process produces a great deal of dust and fume. Due to the logistics of scrap loading and molten metal removal, these furnaces are difficult to enclose. In order to safely use electric arc melting as a decontamination strategy, consideration must be made so that volatile elements and dust are captured and contained. In addition, spent refractory from the furnace hearth and from ladles used to transport molten metal becomes a radioactive waste stream. Slag handling is complex, because radioactive slags must be handled in a molten condition, at or above 1400°C, and slow cooled in such a way that their physical integrity is maintained.
Air Induction Melting
An induction furnace is an AC electric furnace in which the primary conductor generates, by electromagnetic induction, a secondary current that develops heat within the metal charge. Once the charge is molten, this current may also be used to stir the melt. Induction melting permits, but does not require the use of a slag. Induction furnaces are refractory lined, so reactions between the refractory and the metal and the refractory and the slag will occur. Such reactions increase the risk of cross contamination between melts and may cause the presence of contaminated refractory particles in the melt. Molten slag is removed by skimming, often manually. Because these furnaces may be opened for molten slag removal, sealing them is difficult and fumes and dust will escape during the slag skimming process. Induction furnaces favor good melt agitation, relatively easy fume control, and rapid heatup. Induction melting is not as inherently as dusty a process as electric arc melting, producing only 20% as much effluent dust as an electric arc furnace of similar capacity. (3) When used as a melt decontamination strategy, the overall process must include plans for capture of those radionuclides which will report to the fume and dust. Additionally, plans must be made for disposal of contaminated furnace and ladle refractory and for the solidification and containment of spent slag.
Vacuum Induction Melting (VIM)
Vacuum induction melting is based on the same principle as air induction melting, except that melting is performed in a vacuum, facilitating the capture of dust and fume. When performed in a vacuum, induction melting is usually done without a slag layer. Vacuum induction melting is an optimum decontamination strategy for the removal of volatile radionuclides, but, because it is performed without a slag, does not provide a mechanism for the removal of non-volatile radioactive species, except by means of inclusion formation, flotation, and agglomeration.
Electroslag Remelting
Electroslag Remelting, or ESR, is a consumable electrode process in which heat is generated by the passage of electric current through a conductive slag, which is resistively heated. As the electrode melts, the droplets of metal are refined by contact with the slag. The droplets collect at the bottom of a water cooled copper crucible and consolidate to form an ingot with low residual impurities, very few nonmetallic inclusions, and excellent surface quality. ESR produces a metal ingot in the form of a cylinder or a slab, which may be directly formed into a useful product by rolling or forging. Because the ESR process may be controlled remotely, it is easily contained and the volatile radionuclides which are removed from the metal may be effectively captured. During the ESR process, many decontamination mechanisms come into play. Radionuclides may be transferred from metal to slag at three sites: at the molten metal/slag interface present at the electrode tip, at the metal droplet/molten slag interface, and at the interface between the not yet solidified ingot and the slag. In addition, due to the presence of an electric current, two of these surfaces are electroactive, and the flow of electrons may be used to optimize decontamination reactions. Atthe completion of melting, the slag containing the radionuclides is removed as a solid, which is much easier and safer than the handling of liquid slag required by other processes. When performed in its usual mode as a consumable electrode process, ESR requires electrodes to be solid and continuous. Pieces of scrap may be welded together to accomplish this, as long as electrical contact between electrode sections is maintained. Alternately, the ESR process may be used in tandem with another melting process which would consolidate scrap, producing ESR electrodes.
Developmental Studies
Bench scale melting tests for the removal of uranium and transuranics have been conducted by several researchers. At Argonne National Laboratory, Seitz, Gerding, and Steindler (4) studied the decontamination of mild steel, stainless steel, and nickel contaminated with 400-1400 ppm of plutonium oxide. Contaminated metals were melted in a resistance furnace in the presence of silicate slags of various compositions. Several crucible materials were tested to determine their ability to contain molten slag and steel without chemical attack or cracking. Magnesium oxide crucibles were attacked by a calcium silicate slag, while calcia stabilized zirconia crucibles were subject to cracking. The best crucible material tested was recrystallized alumina. After melting, the major source of activity remaining within the ingots was found to be due to the incomplete separation of the silicate slag from the steel. In all experiments performed, the plutonium was shown to be effectively captured by the slag. The chemistry of various slags tested had some effect on plutonium extraction. Partition coefficients (plutonium in slag/plutonium in steel) of 7x106 were measured with borosilicate slag and 3 x 106 for calcium-magnesium-silicate slag. The researchers noted that some of the borosilicate slags were inhomogeneous with higher plutonium and chromium concentrations in regions of high alumina content.
Staged melting was found to improve decontamination efficiencies. Staged melting was done on one heat where portions of two previously melted ingots were remelted using a clean slag. This resulted in an even greater reduction in plutonium content. The initial content of the charge was reported as about 0.1 ppm Pu while the ingot resulting from this melt averaged 0.002 ppm Pu. This reduction was postulated to be the result of the removal of Pu containing inclusions. The possibility was considered that a similar result could have been obtained in the primary melt by a longer holding time in the crucible to allow time for the oxide inclusions to coalesce and float into the slag. The inclusions still trapped in the first melt (which had a duration of one hour) had an additional hour to float to the surface in the second melt. Two other single melts held for two hours under a borosilicate slag yielded similarly low Pu contents, leading to a conclusion that longer holding times may be nearly as effective as staged melting.
In 1978, Copeland, Heestand, and Mateer (5) of Oak Ridge National laboratory published a report which detailed both the theoretical thermodynamics and the practical engineering aspects of planned metal melt decontamination research. In fulfillment of these plans, researchers (6,7,8) studied the effect of slag composition and other processing variables on the degree of decontamination achieved in the melt refining of metals contaminated with UO2. Samples were contaminated by addition of UO2 to the flux, which was added to the metal in a crucible. Melting was then performed at approximately 1600°C in an induction furnace and time was allowed for slag metal equilibration. After cooling, samples were taken of the metal and the slag. The degree of decontamination was not reported to be highly dependent upon slag composition although low efficiencies were associated with high silica slags. In general, highly fluid basic oxidizing slags were found to be more effective in the decontamination of stainless steel.
A logical continuation of the melting work performed at Oak Ridge involved a potential disposal method for the resulting contaminated slags. The feasibility of size-reducing and disintegrating slags was studied. Solidified slags generated in melt refining were crushed and reduced in size to less than 3 mm. The size reduced slags were mixed with cement and found to form stable grout mixtures. (9)
A six ingot scoping study was performed at Hanford (10,11) by Hobbick, Schatz, and Aden who used an induction furnace to make 5 lb. melts. The charge for each melt consisted of five uncontaminated stainless steel bars, two of which were then contaminated as follows: one bar was painted with a slurry containing PuO2 so that the bar contained 0.8 to 1.0 g of Pu (an addition to the total melt of 350 to 440 ppm) and another bar was dipped into an acidified solution of beta-gamma source from Hanford "tank waste" and was thus coated with 60Co, 137Cs, and 155Eu. A flux resembling the borosilicate glass composition was used, but the high SiO2 content caused attack of the MgO crucibles. After melting, autoradiographs showed that the ingot contamination was concentrated on the surface of the ingots and in inclusions. Ingot analysis by wet chemistry showed levels of residual Pu in the 0.1 to 0.085 ppm range, while an approximate 98% reduction of beta, gamma, and actinide levels was achieved.
Uda, Iba, and Tsuchiya (12) investigated the effect of slag chemistry on the melt refining of mild steel. In a related study, Abe, Uda, and Iba (13) melted samples of uranium contaminated mild steel, 304 stainless steel, and copper under various SiO2- CaO-Al2O3 slags. Other variables included in these studies were contamination levels and melting times and temperatures. The aim of this research was to establish an optimized decontamination strategy for various metal types. Rods of the metals to be melted were contaminated by dipping in a uranyl nitrate solution of known concentration and were subsequently heated to decompose the uranyl nitrate UO2 . Samples were then melted in an enclosed furnace under an argon atmosphere. The results of various tests revealed that slag chemistry is an important factor in decontamination efficiency.
The ionic character of a slag may be represented by its basicity, which is defined as the moles of basic oxide (CaO) divided by the total moles of acidic oxide (SiO2 + Al2O3). The most effective slag basicity was around 1.5. Fluxes containing CaF2 were more effective, possibly because as fluorides break the bonds of the silicate network present in SiO2 containing slags, (14) additional SiO44- anions are produced which could combine with uranyl cations at the slag metal interface, causing uranium capture in the slag according to the following reaction:
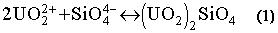
Slags containing NiO also proved to be effective because the oxide breaks down to yield free oxygen ions which also break down the silicate network causing the formation of acid slag anions. Using their optimum conditions, these researchers produced ingots with levels of uranium near the levels of the metal prior to contamination and melting.
In another study of the effect of slag basicity, researchers Ren, Liu, and Zhang (15) conducted 100 gram melt tests in order to determine suitable flux composition and process parameters for the effective removal of uranium from copper, nickel, and mild steel. Using a slag with a composition of 38.1% SiO2, 41.4% CaO, 3.8% MgO, 2.6% Fe2O3, and 14.1% Al2O3 and a basisity of approximately 1.1 as a base, slag basisity was varied from a 0.25 to 1.95. Optimum decontamination was achieved with a flux having a basicity of 1.1.
Research performed at the Montana College of Mineral Science and Technology (16) by Worcester, Twidwell, Paolini, and Weldon involved small scale (two pound) air induction melts of stainless steel contaminated with cerium, lanthanum, and neodymium to simulate the presence of radionuclides. This research showed that the ability of a slag to collect contaminants from a molten metal bath is both time and temperature dependent and thus is kinetically controlled.
Researchers at Kobe Steel, Ltd. and Power Reactor and Nuclear Fuel Development Corporation (17,18) investigated the use of electroslag refining to decontaminate plutonium contaminated waste. This melting method was expected to have advantages as a process for treating radioactive metallic waste. Because melting and solidification take place in the same water cooled copper mold, no refractories are necessary, equipment needs are simplified, and volume reduction and radionuclide immobilization are accomplished simultaneously. Radioactive particles on the surface of the metallic waste can be transferred to the melted slag. Testwork was performed using HfO2 as a surrogate for PuO2, because of the similarity of properties. A 10 kg pilot scale furnace was used to become familiar with ESR operations, after which a 100 kg furnace was constructed and used for simulated decontamination tests. The furnace featured a rectangular copper mold and two 50 mm tungsten bars which served as non-consumable electrodes. The slag used had a composition of 47% CaO, 48% Al2O3, and 5% B2O3 and was selected on the basis of mechanical strength to insure stability in long term storage. The furnace was fed with pieces of surrogate treated metal (67 ppm Hf) by use of a bucket mechanism which moved up and down, receiving pieces of metal in the upper position then descending over the melted slag and depositing the metal into the melted slag. Upon evaluation of both ingot and slag for hafnium content, it was found that the solidified slag containing 298 ppm Hf and the ingot contained 2.53 ppm Hf, yielding a decontamination factor of about 25.
Electroslag refining was also the subject of a study by Uda, Ozawa, and Iba (19) who investigated the potential of this method for melting of uranium contaminated metal cylinders. Electroslag refining was selected because many metallic wastes in the forms of rods, cylinders, and tubes can be melted without cutting them into pieces. The aim of this study was to establish ESR melting conditions for metal cylinders in which the cross section ratio of electrode to mold was less than the usual industrial ratio of 0.3 to 0.6 while comparing the decontamination efficiency of ESR with that for a resistance furnace. The ESR test equipment consisted of a 60 mm water cooled copper mold, an AC power supply, an electrode position controller and an off gas line and filter for removal of fume from the enclosed melting chamber. Cylinders of iron and aluminum were prepared for melting by application of a uranium containing solution to their surfaces so that the resultant uranium concentration was 500 ppm. The flux composition used for the experiments was 40% SiO2 -30% CaO- 20% Al2O3 - 10% CaF2 for the iron, and 14% LiF - 76% KCl - 10% BaCl for the aluminum. The decontamination effect achieved with ESR was better than that obtained with a resistance furnace and was shown to be independent of melt rate. The final ingot concentrations of the iron were 0.01 to 0.015 ppm which were less than the as received specimen before contamination, although ingot levels for the aluminum were 3 to 3.5 ppm which were a few times higher than in the as received condition, before the application of contaminants.
A feasibility study conducted by Atteridge (20) at the Oregon Graduate Institute revealed that the electroslag refining process could be effectively used to directly melt assemblies of stainless steel piping without an initial electrode consolidation step. Using this technology, contaminated reactor piping could be cut to suitable electrode lengths and directly melt decontaminated by ESR. Such a strategy would greatly reduce the need to manually torch cut contaminated material and could have advantages both in terms of processing costs and worker safety.
Full Scale Technical Demonstrations
Stainless steel scrap melting work at the Idaho National Engineering Laboratory (21) was concerned primarily with reducing the volume of the scrap to conserve dwindling waste storage capacity. The Waste Experimental Reduction Facility (WERF) was established to process a variety of low-level beta/gamma contaminated waste. A 750 kW induction furnace was used to demonstrate that melting the scrap and casting into 680 kg ingots was a safe and effective method for achieving at least a 10 fold reduction in volume while converting the scrap into a readily packagable form and stabilizing any radioisotopes remaining in the ingots. The contaminated feedstock was generated by various INEL facilities and consisted mostly of salvaged reactor and test support components that were surface contaminated only. The major contaminants were 60Co, 90Sr, and 137Cs. Because the radioactivity level of the ingots produced had been too low to formulate a basis for the prediction of the dispersion of isotopes resulting from melting, three intentionally contaminated melts were made using sub-sized (200-300 kg) heats. Known quantities of 60Co, 85Sr, and 137Cs were added to uncontaminated stainless steel. 85Sr was used instead of 90Sr because 85Sr is a gamma emitter and therefore easier to detect in minute quantities. After melting, 91-100 % of the cobalt, 0-15% of the cesium, and 0-4% of the strontium remained in the ingots. Most of the strontium was collected in the high silica slag used, while most of the cesium vaporized and was collected by the air filtration system. Distributions in the slag were difficult to measure because slag remained attached to the ingot and the dipping tools, while some reacted with the furnace refractory and remained in the furnace.
The Y-12 plant at Oak Ridge (22) supervised the melting of a 20 year accumulation of low level contaminated metal waste in a 10 ton electric arc furnace; 27000 tons of scrap were melted requiring 2037 heats for the carbon steel scrap and 218 heats for the stainless steel scrap. The mild steel ingots averaged 0.4 ppm with 94.3% of the heats containing 1 ppm or less of uranium while the stainless heats averaged 0.6 ppm with 79.8% of the heats containing 1 ppm or less. The slags for the carbon steel heats averaged 2630 ppm uranium.
In a study performed at Sandia National Laboratory (23) stainless steel bars were coated with non-radioactive surrogate elements in order to simulate surface contamination. These bars were electroslag remelted using slags of various chemistries. The slags investigated were ternary mixtures of calcium fluoride, calcium oxide, and alumina. The final chemistries of the stainless steel ingots were compared with those predicted by the use of a Free Energy Minimization Modeling technique. Modeling also provided insight into the chemical mechanisms by which certain elements were captured by a slag. Slag selection was also shown to have an impact on the electrical efficiency of the process as well as the surface quality of the ingots produced.
The European community has been active in treating radioactive scrap by melting techniques. Lacroix and Talliez published a study of the melting of portions of a rejected carbon steel heat exchanger from the Chinon A power plant. (24) The activity of the incoming metal was between 10 and 20 mCi per metric ton. The purpose of the test was to determine the behavior and final distribution of radionuclides after melting as well as to evaluate the potential contamination of the surroundings due to the melting and casting processes. The study used a refractory lined graphite electric arc furnace of 4 to 5 metric ton capacity equipped with a mobile hood for fume capture. After melting, the total weight of the ingot was 3660 kg. The weight of the slag was 200 kg and that of the dust was 30 kg. The activity of each of the products was measured and 76 mCi was found in the ingots, 217 µCi in the slag, 148 µCi in the filter dust, and 38 µCi in the molding sand. Radiation checks in the vicinity of the furnace during operation revealed that the atmospheric activity did not exceed the maximum permissible level for public exposure of 10 pCi/m3. The report concluded that the dose rates are sufficiently insignificant to permit the operation of a 4000 metric ton operation in complete safety.
British Steel Corporation (25) conducted a study to determine the practicality of releasing properly treated and characterized RSM to commercial electric furnace operations. The scrap used in this study was composed of contaminated steel from three different reactor systems which was mixed in various ratios with uncontaminated steel. The study consisted of 16 heats, six of which were melted in 50 kg or 0.5 tonne induction furnaces and ten of which were melted in a 5 tonne electric arc furnace. The radionuclides of interest were 60Co, 134Cs, and 137Cs. Because of its chemical similarity to iron, the cobalt consistently partitioned to the steel castings while the cesium was captured in the slag and the fume. The partitioning of cesium between slag and fume was reported to be a function of the melting technique, the slag composition and the type of scrap melted. After analysis, all the ingots produced in this study were diluted in 300 tonne basic oxygen furnaces to less than 10-5 µCi/g. In a continuation of this work, experiments were undertaken in which europium (152Eu and 154Eu) contaminated steel was melted in a 500 kg induction furnace as well as in a 3 tonne arc furnace. It was found that the europium was contained wholly in the slag under both oxidizing and reducing conditions and that none was detectable in either the steel or the off gasses. The conclusion drawn from this research was that, although the process could be operated within published safety criteria, the cost of controlling and monitoring the scrap would out weigh its value. This research program was concluded in 1988 with no plans of future work. (26)
Decontamination technology for use in decommissioning the Japan Power Demonstration Reactor (JPDR) has been the object of research by the Japan Atomic Energy Research Institute (JAERI). (27) The objectives of tests performed by JAERI have been to investigate and assess the behavior of radionuclides during melting and casting and their influence on the working environment. Design of test equipment took place in 1987 and installation was completed by the end of March, 1990. (28) The melting equipment includes a 500 kg high frequency induction furnace equipped with an air tight hood. Melting and casting equipment are enclosed in a steel chamber which is kept at a slightly negative pressure to minimize the spread of radionuclides. Following equipment installation, cold tests on non-radioactive carbon and stainless steels were performed.
By September 1991, two heats of 304 stainless steel and four heats of carbon steel recovered from JPDR had been conducted. A series of five stainless steel test melts were performed using gamma emitting tracers as surrogates for nuclides commonly found in contaminated material from light water reactors, 54Mn, 60Co, 65Zn, 85Sr, and 137Cs. In a second series of tests, 63Ni was used as a beta emitting radionuclide. Experimental variables included the type of contamination, the CaO/SiO2 ratio in the flux, and melting temperature. The study examined the work place radiation dose, radionuclide partitioning among ingot, slag, dust, and offgas, and the final distribution of radioactivity within an ingot. It was found that 99.5% of the cobalt, 91% of the manganese, and 75% of the zinc remained in the ingot and were uniformly distributed. None of the strontium or the cesium was found in the ingots. Slag analysis showed that no cobalt was present, while 1% of the zinc, 7% of the manganese, 39% of the cesium, and 73 % of the strontium were present. Accuracy of the fume analysis was questioned because 7% of the zinc, 27% of the strontium, and 23% of the cesium remained unaccounted for. This problem could have been caused by condensation of the fume before it reached the sampling location. (29)
Industrial Operations
Jacquet-Francillon and co-researchers (30) reported the development of a cold crucible induction melting operation designed to melt contaminated zircaloy and stainless steel hulls from the pilot nuclear facility at Marcoule. An industrial scale facility designed to process 15 ingots/year began production in 1993. In addition, a three phase, 15 tonne arc furnace has been used since April of 1992 for the melt consolidation for the treatment of ferrous materials recovered from the dismantling of the CO2 systems from the graphite moderated, CO2 cooled plutonium production and power generation reactors at Marcoule. By April 1993, an estimated 2580 tonnes of cast iron had been produced for reuse within the nuclear industry. (31,32)
Melt consolidation of ferrous scrap from dismantling nuclear power plants began at the Siempelkamp foundry in Krefeld, Germany in 1984. (33) While the first melts were performed using a modified 20 tonne coreless induction furnace, a new facility became operational in 1990. This facility consists of a 3.2 tonne 300-500 Hz coreless induction furnace with a melting capacity of 2 tonne/hr. The furnace facility is equipped with a fume system which provides negative pressure to the melting enclosure. (34) This facility was used to melt 500 tonnes of steel scrap from decommissioning the Gundremmingen Unit A (KRB A) nuclear power plant. Mies (35) reports that samples taken from remelted scrap are within permissible levels of activity, that the slag is disposed of at normal industrial waste facilities, and the filter dust is stored as low level waste.
In Sweden, radioactive scrap metal of Swedish and German origin was melted at Studsvik AB. By the end of 1988, over 400 tonnes of low activity scrap metal had been melted, of which approximately 90% was carbon steel and the remainder was stainless steel. The induction furnace employed has a capacity of 1.5 tonne/hr. Analytical results showed that Co, Mn, Zn, Ag, and Sb remained in the steel while Cs partitioned to the slag and dust. (36)
In the United States, Scientific Ecology Group, Inc. (SEG) (37) currently performs RSM consolidation in a 20 ton, 7200 kW induction melting furnace. During one melting campaign, 2735 tons of carbon steel material was melted and cast into shield blocks. In a second campaign, 2200 tons of carbon steel was consolidated into shield blocks for nuclear operations. (38)
Recycling and Reuse of Decontaminated Metal
While many countries have recently begun melting of radioactive scrap metal for densification and limited reuse for low technology applications, there is, as yet, no plan in place for the recycle of specialty metals for high technology applications. The stockpile of contaminated metals in the United States is composed of valuable alloys which could be decontaminated and reused for nuclear related applications with their original properties intact. The alternative is the costly disposal and long term monitoring of these materials. The choice to dispose of contaminated strategic metals leads to the use of virgin materials for use in nuclear applications. These virgin materials will become contaminated, adding to the problem. If, however, the alternative of decontamination and recycle is chosen, storage costs are greatly reduced and the need for materials to be used in present or future nuclear applications is met.
It is theoretically possible to simply release melt decontaminated RSM into the non-nuclear community scrap metal market place, and this is being done overseas. However, present use-restriction rules in the US do not allow remelt RSM to be mixed with non-radioactively contaminated scrap, no matter how low the level of residual contamination. These rules dictate that RSM scrap remain separate, and not be used for general domestic/industrial use. It can, however, be used for nuclear community-specific applications, and, indeed there are multiple possible uses within the nuclear community for remelted RSM. These vary from low-tech waste burial boxes and barrels to high-tech transportation and interim/permanent waste storage canisters for high level radioactive waste.
SUMMARY
Significant research in the field of melt decontamination has demonstrated that, where appropriate, these technologies represent a viable method for the recovery of contaminated metallic assets. The availability of these technologies makes the burial of such contaminated metal, often containing strategic elements, a senseless and unnecessary waste of money and resources. Therefore, in light of the technology and research available, it is perhaps time to review present disposal options and techniques with a greater eye toward asset recovery.
REFERENCES