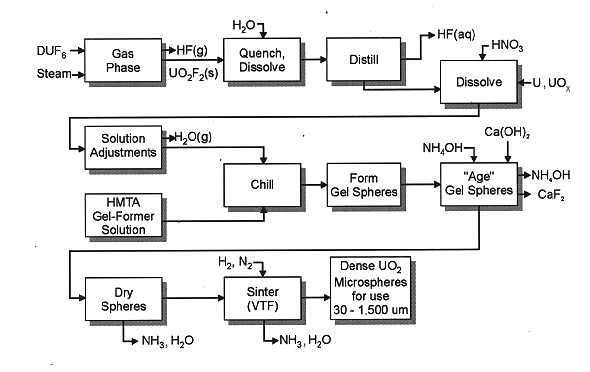
Fig. 1. Overview of the Gelation
Process for Producing Dense Uranium Dioxide from DU
Alexander P. Murray, Steven M. Mirsky, and Stephen J. Krill,
Jr.
Science Applications International Corporation (SAIC)
20201
Century Boulevard
Germantown, Maryland (USA) 20874
ABSTRACT
Depleted uranium (DU) exists at many nuclear fuel cycle facilities around the world. DU is a byproduct from enrichment operations, and, while not a waste, no country currently has plans for large-scale recycle and reuse of DU. This paper presents several potential uses of DU, and concludes that any significant reuse requires a high density form, typically over 8 g/cc. Dense, nonmetallic forms of DU as shielding represents the most significant reuse opportunity. This paper presents a new, integrated, large-scale approach for producing such shielding. The approach accepts the DU as either the hexafluoride, oxide, or the metal, and produces a dense, uranium dioxide based shielding material using an internal precipitation technique. Waste streams and quantities have been estimated. Designs and layouts for the manufacturing facility have been generated. The paper presents preliminary cost information for several plant designs with capacities of 5,000 to 30,000 tonnes (U) per year.
The paper determines that shielding requirements for SNF represent the largest potential application and would easily consume the existing inventory of DU in the United States. The conversion process does not require unusual reagents and equipment, and all input and construction materials are available from existing and established markets. The paper estimates similar size and waste disposal volumes as experienced by existing, fuel fabrication plants, even though the DU throughput is twenty-times larger. The economic analyses clearly demonstrate the economies of scale achieved by larger plants, allowing finished product cost of $5-6 per kilogram of dense DU dioxide. The use of DU in SNF containers pays for the processing costs. The paper concludes that this represents a cost effective approach for using the stored inventory of DU in an environmentally compatible manner.
INTRODUCTION
The nuclear industry generates large quantities of depleted uranium (DU) as a byproduct of processing and enrichment operations. In the United States, for example, approximately 500,000 tonnes (U) of DU exist. DU hexafluoride from enrichment constitutes approximately 90% of this material, while the remainder is split between oxide and metallic uranium forms. The DU materials are currently stored at domestic and overseas fuel cycle facilities, and, ideally, both processing methods and applications for DU are desired. A significant quantity, perhaps in excess of 90%, of the DU is stored as the hexafluoride, in mild steel cylinders. For example, the United States currently has an inventory of DU hexafluoride approaching 600,000 tonnes (1). DU metal and oxide constitute essentially all of the remainder. Consequently, any DU recycle and reuse opportunities must accommodate the processing of all three materials as feedstock.
This paper presents potential uses for DU, processing routes, and design parameters and economics for a large-scale route for dense, uranium dioxide manufacture. The dense DU dioxide is suitable for subsequent use as radiation shielding.
POTENTIAL USES AND MARKETS OF DEPLETED URANIUM
Reference 2 presents options for the management of depleted uranium hexafluoride. All of the reuse options require depleted uranium in a dense form, with bulk densities in excess of 8 g/cc. The reference identifies DU-based radiation shielding as the principal reuse option for the next few decades. Dense uranium dioxide and uranium metal are the two principal chemical forms for shielding. Calculations estimate significantly lower environmental, safety, and health impacts from manufacturing dense uranium dioxide shielding as compared to uranium metal based shielding (3). Furthermore, high density uranium dioxide is compatible with storage and disposal, and reduces their impacts.
The domestic U.S. market for shielding may grow rapidly due to the increasing quantities of spent nuclear fuel (SNF) from commercial power reactors and the initiation of high-level waste (HLW) vitrification by the U.S. government. In addition, the U.S. Court of Appeals recently upheld the U.S. government's obligation to start accepting commercial SNF in January 1998, as required by the Nuclear Waste Policy Act. Storage and disposal account for most of the containers and shielding needs. The SNF container market exceeds 10,000, based upon current and proposed SNF cask designs. HLW designs are more conjectural, but several thousand casks appear to be needed for the anticipated, 15,000 to 25,000 canisters of vitrified HLW. These numbers translate into a potential market for the next few decades exceeding 500,000 tonnes of uranium dioxide based shielding if DU is used.
Consequently, an economical route to large scale production of uranium dioxide and related compounds is desirable as the initial step towards DU shielding manufacture and use.
POTENTIAL ROUTES TO DENSE URANIUM DIOXIDE
Currently, dense uranium dioxide is almost exclusively used in nuclear fuel applications. Powder processes provide the basis for the high-volume production of this fuel, and annual production amounts to approximately 4,000 tonnes/yr in the United States (4,5,6). The manufacturing route starts with enriched uranium hexafluoride. This material is defluorinated by either aqueous precipitation/calcination (e.g., the precipitation of ammonium diuranate - ADU) or steam calcination at elevated temperatures (i.e., the "dry" routes) to produce a low density, uranium dioxide powder (typically, 3-4 g/cc). Subsequent powder processing includes mixing, granulation, pellet pressing, sintering, pellet grinding, and fuel rod loading. In use, the powder-process based plants are modular, using operations based on typical module capacities around 100 tonnes/yr. Such an operating level works well for nuclear fuel, but appears impractical for large scale shielding use; a DU processing capacity equivalent to all current U.S. uranium dioxide plants would require 150 years to eliminate the current DU inventory alone, and scaleup to the potential requirements of DU use would necessitate hundreds of lines.
Practical production of dense, DU dioxide necessitates improved, higher throughput, manufacturing methods. Fortunately, alternate methods for dense uranium dioxide manufacture were investigated in the 1960's, 1970's, and 1980's to avoid the labor and mechanically intensive, powder processes (1,7,8,9). These alternate processes are termed "gelation," and have various terminologies and synonyms like sol-gel, gel-precipitation, internal gelation, external gelation, particle fuel, microsphere, and solution precipitation. All of these processes use hydrodynamics to form spheres of ADU, which are subsequently cured, dried, and sintered into high density, uranium dioxide microspheres (typically, 95-98% of theoretical density). Adjustment of operating conditions allows the production of microspheres over the 33 to 1,500 micron size range. The process can be tailored to produce a narrow size distribution, coated spheres, and uranium carbide. At the time, the intention was to apply gelation processes for the production of mixed oxide fuels, and for the expansion of capacity at existing fuel fabrication plants. However, the abandonment of nuclear fuel recycling in the U.S. and the lower than anticipated growth in the nuclear industry did not provide for the implementation of gelation methods beyond pilot plant evaluations.
THE GELATION PROCESS
Gelation methods represent chemical processes and are more amenable to scaleup at the 10,000-30,000 tonne annual throughputs postulated for the potential market. Figure 1 outlines the internal gelation route used as the basis. Some 90% of the DU feed is the hexafluoride; this is volatilized and reacted with near stoichiometric quantities of steam at low temperatures to produce uranyl fluoride and high quality hydrofluoric acid (HF). The HF is condensed and recycled as a clean material due to its extremely low DU content (circa 0.2 ppm or less). The process quenches and dissolves the uranyl fluoride.

Fig. 1. Overview of the Gelation
Process for Producing Dense Uranium Dioxide from DU
Nitric acid dissolves metallic and oxide forms of DU in reaction kettles, producing uranyl nitrate. Hence, the common stream from the feed area becomes a uranyl nitrate/uranyl fluoride solution. The uranium solution is clarified, and urea is added for uranium stabilization and complexation. The process concentrates and chills this solution, and then combines it with hexamethylenetetramine (HMTA). In chilled solutions, the mixture is stable. However, upon heating, the HMTA partially decomposes and produces ammonia, which precipitates the uranium as ammonium diuranate (ADU). The gelation uses this property to its advantage by dispersing the chilled solution as fine droplets into an immiscible, warm (50-90°C) oil medium. As the droplets fall through the oil bath, they assume the shape of least resistance, namely a sphere. Simultaneously, the ammonia from HMTA decomposition precipitates ADU, which strengthens and hardens the droplet. After aging in the oil bath, the spheres are removed and washed with ammonium hydroxide, which completes the ADU precipitation. Warm nitrogen dries the spheres, producing initial densities of 3-4 g/cc. Sintering occurs in a moving bed within a vertical tube furnace, and results in particle densities exceeding 10 g/cc and bulk densities of around 9 g/cc.
The design considers an integrated process at a stand-alone site. The approach recycles reagents where practical and includes waste management through solidified wasteforms, ready for shipment to a disposal site. Figure 2 displays the overall mass and energy balances.
FACILITY DESIGNS
Facility designs have been generated for plants with uranium dioxide capacities from 5,000 through 30,000 tonnes/year. All designs assume a "greenfields" site and stand-alone operation, and group the buildings into four basic types:
The discussion focuses on the main building, which houses the major equipment for the gelation process. As shown in Fig. 3, the main building includes kiln and dissolution areas for producing the uranyl nitrate main feed stream. The gel forming and drying areas include stainless steel columns and recirculation equipment, and represent around 60% of the building. Gel forming occurs in columns some thirty feet high. The design assumes the production of two different sizes of spheres, with nominal diameters of 1,100 and 400 microns, and a 70/30 production split, respectively. Sintering occurs in electrically heated, vertical tube furnaces. Streams are purified and recycled; in particular, the HMTA and urea essentially function as homogeneous catalysts, and makeup requirements are modest. The design estimates the main building's footprint as 13,000 m2 (140,000 ft2); the remaining buildings increase the total facility footprint to approximately 65,000 m2 (700,000 ft2). Over 30% of this area is used for waste management, storage, and utilities.
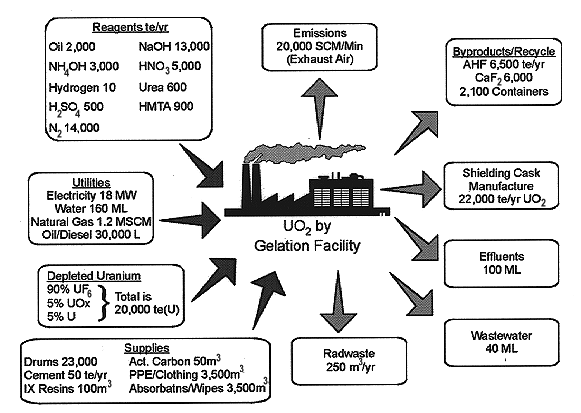
Fig. 2. Overall Mass and Energy
Balances

Fig. 3. Main Building Design
Plant operations involve the volatilization and handling of gaseous uranium hexafluoride, via a "warm feeding" approach that does not liquefy the hexafluoride first. This avoids the cylinder pressurization and potentially larger accident source terms associated with the liquid. However, NRC criteria (10) indicate minimum distances to the public of 500 m (about 1,600 ft) due to the handling of gaseous uranium hexafluoride. This results in a minimum site size of about 300 hectares (750 acres).
SCALING AND ECONOMICS
Economic analyses are being conducted over the 5,000-35,000 tonne/yr capacity range. Figure 4 presents the results. The unit cost is very reasonable over the entire capacity range as compared to current, fuel pelletization routes. However, the unit cost increases rapidly below a 10,000 tonne/yr capacity, and, thus, large plants are clearly favored. Above a capacity of 20,000 tonnes/yr, the slope, and, hence, the unit cost reductions, become marginal. A 10,000 tonne/yr capacity approximately equals a 50 year operating period for the current DU inventory in the United States. The two inflection points arise from step function changes in several of the cost contributors. Overall, unit costs are in the $5-6/kg bracket for dense uranium dioxide with a greenfields plant. This allows the material to be competitively used in radiation shielding, with the price of the shielding paying for the conversion costs. Production of coated uranium carbide approximately increases the unit costs by 12%. Colocation of the plant at an existing, fuel cycle site (such as an enrichment plant) is beneficial, and offers around a 20% savings for the dioxide and a 15% savings for the carbide.
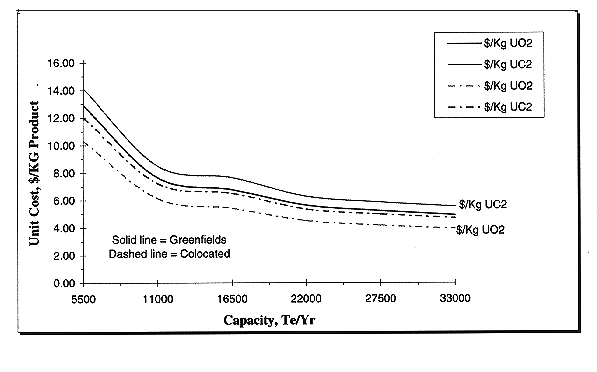
Fig. 4. Cost versus Capacity for
Dense DU Dioxide Manufactured by the Gelation Process
The cost analyses assume handling and transportation charges for the DU materials, but with a DU purchase price of zero. This is a common assumption for DU processing because the DU price was included in the original price of its parent, natural uranium. Hence, the DU is already "purchased." Any additional purchase price will increase the cost by a corresponding amount.
POTENTIAL DEVELOPMENTS AND FUTURE ENHANCEMENTS
The gelation process is inherently flexible. As an example, the gelation process provides a quick and relatively simple route for uranium carbide manufacture, which requires four changes to the basic process:
Notice that a gelation process provides the capability for coating uranium dioxide, thus producing an inherently more stable, micro and macroencapsulated material.
The flexibility of the gelation process also allows for the use of alternate precipitation methods. One advanced scheme under development reduces the basic plant size by 50%, resulting in a significantly smaller plant, reduced capital outlays, and lower costs.
SUMMARY AND CONCLUSIONS
A process and facility design have been generated and evaluated for converting DU from multiple sources into dense uranium dioxide for radiation shielding use or long term storage. The approach converts the DU forms into a common feed of uranyl nitrate and fluoride solution, which is subsequently treated by gelation methods to produce a dense, spherical product of uranium dioxide. The unit costs are in the $5-6/kg range for a greenfields plant, with estimated savings of 20% at a colocated fuel cycle facility, such as an enrichment plant. This compares extremely favorably with other uranium dioxide and trioxide conversion processes, and allows the material to be competitive for large-scale use in radiation shielding. The gelation process produces a nuclear fuel grade product, and is not optimized for shielding or storage purposes. Therefore, additional cost savings are anticipated. More analyses are planned.
REFERENCES