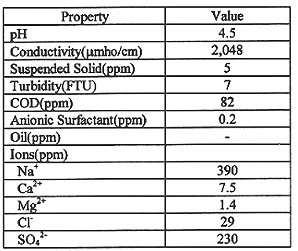
Table I Chemical and physical properties of liquid radwaste.
AN APPROACH TO LOW-LEVEL LIQUID RADWASTE TREATMENT BY ION EXCHANGE
Sung Paal YIM, Kil-Jeong KIM, Joon-Hyung KIM
Korea
Atomic Energy Research Institute
Nuclear Environmental Management Center
P. O. Box 105, Yusong, Taejon, 305-600, Korea(KOR)
Jong-Kil PARK, Myung-Jae SONG
Korea Electric Power
Research Institute
#103-16, Munji-Dong, Yusong, Taejon, 305-380, Korea(KOR)
ABSTRACT
The interest to low-level liquid radwaste treatment by ion exchange is increasing in Korea. A project to develop an ion exchange process has been performed in cooperation of Korea Atomic Energy Research Institute(KAERI) with Korea Electric Power Research Institute(KEPRI). As a part of this project, the ion exchange method has been applied to treat low-level liquid radwaste stored at RWTF. This paper presents the applicability of the ion exchange approach to treat liquid waste, and demonstrates the laboratory and pilot scale test at RWTF.
INTRODUCTION
The evaporation method has been utilized to treat low-level liquid radwastes produced from the PWRs in Korea. This method generally leads the decontamination and volume reduction of liquid waste. However, at nuclear power plants, chemicals and impurities contained in liquid radwastes raise unwanted problems such as corrosion, scale, and a foaming during evaporation, etc. These problems may markedly decrease the ability of decontamination. Besides, the volume reduction occurred by the evaporation process is limited due to characteristics of boric acid in the liquid waste, which have property to be crystallized above a certain concentration.
Radwaste Treatment Facility(RWTF) at Korea Atomic Energy Research Institute(KAERI) also employed the evaporation method. Liquid waste is concentrated by evaporator and then its concentrate is solidified by bituminization. Currently, the performance of evaporator is satisfactory in decontamination, volume reduction and operation. However, if unexpected large amounts of liquid waste happen to be transferred from other facilities to the RWTF, it makes evaporator overloaded temporarily. Nevertheless, neither the additional installation of evaporator nor the extension evaporator capacity has been considered as appropriate counterplan because of the technical and economical viewpoint
In order to deal these problems, a project to develop an ion exchange process has been performed in cooperation of Korea Atomic Energy Research Institute(KAERI) with Korea Electric Power Research Institute(KEPRI). As a part of this project, the ion exchange method has been applied to treat low-level liquid radwaste stored at RWTF. This paper presents the applicability of the ion exchange approach to treat liquid waste, and demonstrates the laboratory and pilot scale test at RWTF.
PROPERTY OF LIQUID RADWASTE
The chemical and physical properties of liquid waste treated at RWTF are shown in Table I. With reference to the table, liquid waste sampled had a mean pH of 4.5 and contained sodium ion and sulfate ion as the dominant cation (390ppm) and the dominant anion (230ppm), respectively. As shown in Table II, the dominant radioactive nuclides in liquid waste were 60Co, 134Cs, and 137Cs, but iodine was not detected.
Table I Chemical and physical properties of liquid radwaste.

Table II Radioactive nuclides and activity in liquid radwaste.

It has been reported that cobalt may exist as cation, colloid or insoluble
matter, while cesium exists as cation in most liquid radwastes.(1) The
radioactivity of liquid waste has been measured with the multi-channel
analyzer(MCA) installing pure Ge detector after filtration with a 0.2
![]() m
filter. The activity of each nuclide was indicated to be unchanged. Considering
this result, it is appeared that most of cobalt existed as ion in liquid waste.
m
filter. The activity of each nuclide was indicated to be unchanged. Considering
this result, it is appeared that most of cobalt existed as ion in liquid waste.
ION EXCHANGE MATERIALS
In order to select an appropriate ion exchanger, several kinds of materials that are commercially available on treatment of liquid radwaste were tested and evaluated. Specification of materials are summarized in Table III. Activated carbon was tested if it has ability to remove a large portion of cobalts from liquid waste and synthetic zeolite and porous glass was examined for cesium removal. Organic resins was determined for both cobalt and cesium removal.
Table III Specification of ion exchangers used in experiment.
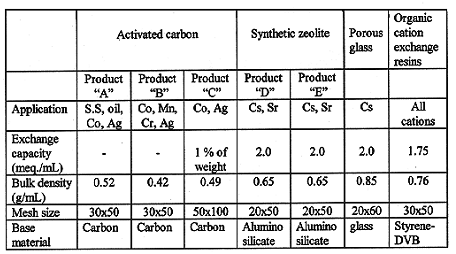
There are various kinds and characteristics of activated carbon, synthetic and natural zeolite commercially available. Three kinds of activated carbons (A, B, C) manufactured for treatment of liquid radwaste were selected for the laboratory scale test. Two kinds of synthetic zeolite(D, E) and a porous glass were chosen for the test. Organic resins is a common material in the field of nuclear industry. The typical strong acidic cation exchange resins, gel type H+ form 8% cross linked sulfonated styrene-DVB copolymer, was selected in this study.
LABORATORY SCALE TEST
Batch Test
In order to observe the removal capacity of ion exchanger for radionuclides at equilibrium, the batch equilibration tests of all ion exchangers were conducted on sampled liquid waste. An aliquot of ion exchanger was placed in sealed polyethylene flask containing a fixed volume of liquid radwaste. The mixture was stirred a prescribed time. When equilibration was completed, the ion exchanger and supernatant solution were separated by filtration. The activity of supernatant solution was measured by MCA with pure Ge detector. Relationship between the activity in ion exchanger and residual activity in solution is then plotted on logarithmic graph. The data for cesium removal is shown in Fig 1. The figure represents that removal capacity of synthetic zeolite is superior to that of porous glass and organic ion exchange resins. Selectivity of synthetic zeolite on cesium is higher than that of porous glass and organic cation resins in relatively high concentration of sodium ion.
Table IV. Bed volumes at 1% breakthrough point for 137Cs

Fig. 1. Comparison of removal capacity of ion exchangers for 137Cs.
Figure 2 illustrates the results of the removal capacity comparison between organic ion exchange resins and activated carbon for cobalt in liquid waste. Organic cation exchange resins has the higher removal capacity than activated carbon. Activated carbon illustrated was the same material, a product "A," as described in Table III. Because the removal capacity of other activated carbons, products "B" and "C" are relatively lower than that of product "A," the data for them are not plotted here. Organic cation exchange resins shows the high selectivity for cobalt, unlike for cesium, in the condition of relatively high concentration of sodium ion. The reason is assumed that affinity of divalent ion like cobalt ion is stronger than that of monovalant ion like sodium ion on organic cation exchange resins.

Fig. 2. Comparison of removal capacity of ion exchangers for 60Co
Although activated carbon is not appropriate for cobalt removal in liquid waste, it plays an important role in decreasing COD of liquid radwaste. Figure 3 shows the effect of activated carbon "A" on decrease of COD in liquid waste.

Fig. 3. Effect of activated carbon on decrease of COD in liquid radwaste.
Small Column Test
Small column test was carried out using the glass column(2.5 x 45 cm). Volume of the ion exchanger was 15 cm3. The liquid radwaste was fed in the flow rate of 3 ml/min. The activity of samples collected from influents and effluents were compared and analyzed. Breakthrough point was established at the point of 1 % leakage for cesium and 10 % leakage for cobalt. Ion exchanger used in this test were activated carbon, synthetic zeolite and organic cation exchange resins.
Table IV shows the result for removal of cesium in small column test. The 1% breakthrough point for cesium reached about 2,700 bed volumes in case of synthetic zeolite while the point was 180 bed volumes in case of organic cation exchange resins. Table V shows the result on removal of cobalt. The removal capacity of organic resins was about 3,000 bed volumes at 10 % breakthrough point and that of activated carbon was less than 80 bed volumes. From the results of these tests, it has been considered that cesium and cobalt in the liquid radwaste could be effectively removed using synthetic zeolite and organic cation exchange resins.
Table IV Bed volumes at 1 % breakthrough point for 137Cs.

Table V Bed volumes at 10% breakthrough point for 60Co.

PILOT TEST
As final step of this study, the pilot test was prepared and conducted at RWTF. This step was to demonstrate effectiveness of ion exchange approach in treatment of liquid radwaste.
Pilot Plant
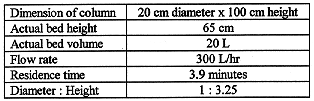
The process flow diagram of pilot plant is shown in Fig. 4. The main components of pilot plant were a liquid radwaste feeding tank, a liquid radwaste feeding pump, a filter unit, and four ion exchange columns. The detailed specification of each ion exchange column is shown in Table VI. The another component was a system for feeding and discharging of ion exchangers, which equipped with a ion exchanger feeding tank, a ion exchanger feeding pump and a spent ion exchanger receiving tank.

Fig. 4. Process flow diagram of pilot plant.
Table VI Specification and operating condition of ion exchange column.
Some sensors and indicators were also installed to measure temperature, pressure and flow rate. All processes involved in operation were controlled from the control panel. For the safety, the front of pilot plant was shielded with 5 cm thick lead brick.
Operation Condition
Four columns were packed with an activated carbon, a synthetic zeolite, an organic cation exchange resins and an organic anion exchange resins. The packed volume of a material in each column was 20 L. Liquid waste passed through these four columns in order. The flow rate was controlled at 300 L/hr. Influents and effluents of each column were analyzed periodically.
Results
Approximately 60 m3 liquid radwaste was treated by the pilot plant. During the test, activity of 137Cs and 60Co in effluents could be maintained under 2.3 ×10-6 mCi/mL and 5 ×10-6 mCi/mL respectively. Decontamination factor at the end of operation indicated 2,400 for 137Cs and 62 for 60Co. The result of pilot test is summarized in Table VII. The columns of synthetic zeolite and organic cation exchange resins could treat up to 2,830 bed volumes and 3,100 bed volume of liquid radwaste, respectively. This result is almost the same as that obtained from the laboratory scale test. Besides this pilot test, another test using a organic cation exchange resins was carried out at the same condition. In that test, only 230 bed volumes of liquid radwaste could be treated by organic cation exchange resins due to leakage of cesium in spite of no leakage of cobalt. It was shown that the amount of liquid waste treated by the use of both of synthetic zeolite and organic cation exchange resins was about 8 times more than that treated by an organic cation exchange resins. Accordingly, it was confirmed that the zeolite and organic cation exchange resins were considered as the desirable media to treat liquid radwaste in ion exchange method.
Table VII Summary of pilot test results.
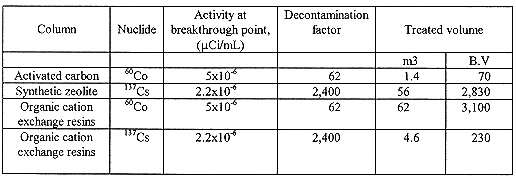
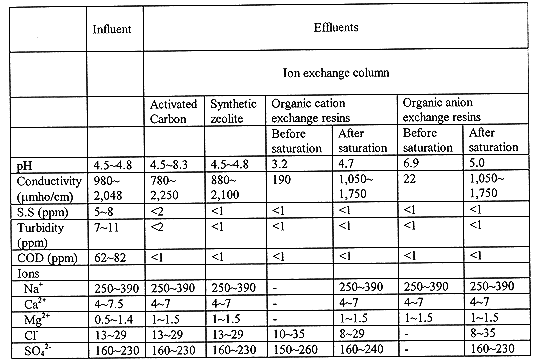
Table VIII shows the chemical and physical properties of effluents. The general quality of effluents was improved by the use of activated carbon and organic anion resins. Activated carbon was not effective to remove cobalt. However, it could protect the other ion exchangers and increase the quality of effluent as it can remove the organic matters. Organic anion exchange resins was used to control the pH of effluent from organic cation exchange resins column. Its role would be more important if iodine existed in liquid waste.
Table VII Properties of effluents.
SUMMARY
Liquid radwaste to be treated at RWTF contains relatively high concentration of salts. The major active nuclides in liquid waste are analyzed as 60Co, 134Cs and 137Cs. Conventional ion exchange method using organic cation and anion exchange resins is not desirable to treat this liquid waste because much volume of spent resins is generated. The major reason is that the removal capacity of organic cation exchange resins for cesium is affected by monovalent ion like sodium ion. However, the removal capacity of organic cation exchange resins for cobalt is hardly affected by monovalent ion like sodium ion. Organic cation exchange resins is useful to remove just cobalt in liquid radwaste containing relatively high concentration of sodium ion. Some commercial synthetic zeolites has the high selectivity for cesium under condition of relatively high concentration of sodium ion. Therefore cesium and cobalt in liquid radwaste can be effectively removed by ion exchange using synthetic zeolite and organic cation exchange resins, respectively. These facts were confirmed by the laboratory and pilot scale test in this study. It is concluded that ion exchange method using synthetic zeolite and organic ion exchange resins is very desirable on liquid radwaste treatment at RWTF in the view of the volume of spent resins and cost of operation. These results are coincide with reports of Electric Power Research Institute(EPRI).(1,2)
Considering the results of this study, the developed ion exchange process to treat liquid waste is planning to apply to nuclear power plants.
REFERENCES