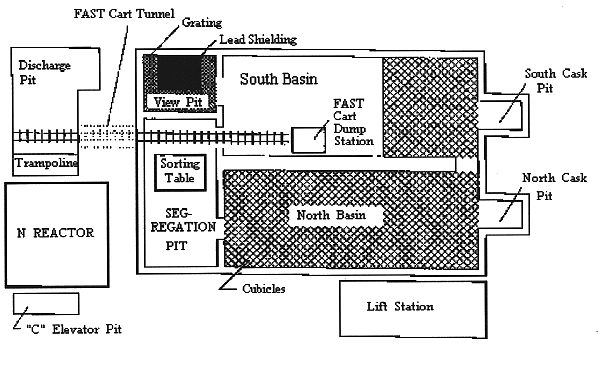
Fig. 1. NBasin plan view.
Theodore D Anderson
BHI
James B. Duncan
WHC
Julian E Laurenz
CHI
ABSTRACT
The N Reactor fuel storage basin (NBasin) holds approximately 1.1 million gallons of radioactively contaminated water along with debris and hardware remaining from reactor operations which ceased in 1989. The water contains dissolved and suspended solids, varying levels of algae and bacteria, and suspected chelated colloidal metals. The bottom is covered with a layer of sediment averaging about one inch deep. Equipment and debris are to be removed and the sediment isolated in the North Cask Pit associated with the Basin. After removing debris, equipment and sediment, the water will be transported to the Hanford 200 Area Effluent Treatment Facility (ETF), the sludge will be sent to the Hanford Tank Farms and the basin stabilized for subsequent decommissioning.
Water clarity is important to basin operations for the removal of equipment, debris and sediment. In the years since shutdown, clarity has varied from good to quite turbid. In March 1996, an aggressive campaign was begun to identify and treat causes of turbidity in Basin water. Testing and measurement equipment was procured, and a formal system of water treatment begun. Initial problems with algae and bacterial growth were solved and recommended pH and chlorine levels established and maintained. Although clarity improved, it was irregular and insufficient. A rigorous approach was then instituted to find appropriate water treatment chemicals to coagulate and flocculate turbidity inducing materials which likely include chelated colloidal metals. It was recognized that because of the complexity of the water chemistry, the specific treatment selected was likely to be unique. A further challenge in reducing turbidity is that the water must be treated in-situ within the confines of the basin walls while debris and equipment removal activities are proceeding.
Approximately 60 jar tests were carried out to determine effective polymer combinations and dosages. Coagulation and flocculation polymers Cat Floc TLTM and POL-E-ZTM were identified as being efficacious to the reduction of turbidity and rendering a high shear flocculent at treatment concentrations of 50 and 10 ppm respectively. In addition it was revealed that strontium was being precipitated out of the water. A small bench scale model, using a high shear pump, was constructed, using in line mixers to enhance charge neutralization and flocculation. Over 45 bench tests allowed optimization of polymer concentrations to 40 and 5 ppm using solutions of 5% and 1% Cat Floe TLTM and POL-E-ZTM respectively. These tests also provided a basis for selecting equipment to treat the Basin water.
Equipment is being modified and/or procured to treat Basin water in the fall of 1996. Among other issues remaining to be addressed are the acceptability of the polymers and degradation products to the Effluent Treatment Facility and the Tank Farms as well as the effects on the safety basis of any redistribution of radionuclides resulting from the water treatment.
INTRODUCTION
The N Reactor spent fuel storage basin (Nbasin) is a reinforced concrete containment basin designed to store and contain spent fuel elements, irradiated spacers, and other fuel handling equipment (Fig. 1 ). Nbasin began operation in 1963 and ceased storing irradiated nuclear fuel in 1989 when all fuel was transferred to the 100K fuel storage basins.
As a result of Nbasin operation, a significant amount of radioactively contaminated equipment, debris (hardware), and residual material (sediment) remains on the basin floor. Nbasin stabilization activities are required to minimize environmental contamination potential by removing the hardware, sediment and over 1.0 million gallons of water. Stabilization was in progress, but was slowed down considerably when basin water visibility diminished. Water visibility problems are mainly the result of biological growth together with suspended and colloidal solids. Biological growth is controlled through the use of chemicals; however, suspended and colloidal solids have remained a problem.

Fig. 1. NBasin plan
view.
Initial attempts to control suspended solids were made through mechanical methods. These included filtration (both localized and overall), and skimming but were not effective in improving visibility for long periods or over the entire Nbasin. Therefore, it was decided to pursue more aggressive methods to obtain and maintain water clarity using coagulants and/or polymers. Both jar tests and bench scale experiments were planned and successfully executed. The jar tests would determine the appropriate coagulant and/or polymer to use for the bench scale experiments. The bench scale experiments would then provide key information for basin treatment application.
THEORY
Coagulation is the addition and rapid mixing of an electrolytically active material, resulting in destabilization of colloidal and free suspended solids, followed by the initial aggregation of the destabilized particles. Flocculation is the use of additional electrolytically active material followed by slow stirring or gentle agitation to aggregate the destabilized particles and form a rapid-settling precipitate.
Colloidal Characteristics
Colloids have a particle size ranging from one millimicron (10-6 mm) to one micron (10-3 mm). An important feature of a solid colloid dispersed in water is that the solid particles will not settle by gravity. When a solid colloid stays in suspension and does not settle, the system is in a stable condition.
Colloidal particles have electrostatic forces that are important in maintaining their dispersion. The surface of a colloidal particle acquires an electrostatic charge due to the ionization of surface groups and the adsorption of ions from the surrounding solution. In general, most naturally occurring colloids have a negative charge if the pH is at or above the neutral range. In most colloidal systems, the colloids are maintained in suspension (stabilized) due to the repulsion of electrostatic charges. A charged colloidal particle will attract counterions from the surrounding water to its surface. The compact layer of counterions is termed the fixed layer; outside the fixed layer is a diffused layer. Both layers will contain positive and negative charged ions; however, if the colloid has a negative charge, there will be a much larger number of positive ions than negative ions. The concentration of the counterions is greatest at the particle surface; it decreases to that of the bulk solution at the outer boundary of the diffused layer.
Colloidal stability depends on the relative magnitude of the forces of attraction and the forces of repulsion. The forces of attraction are due to van der Waals' forces, which are effective only in the immediate vicinity of the colloidal particle. The forces of repulsion are due to the electrostatic forces of the colloidal dispersion. Also, the presence of a bound water layer and its thickness affects colloidal stability, since it prevents the particles from coming into close contact.
Charge Neutralization
Charge neutralization can be achieved by two different types of chemicals: 1) a coagulant such as aluminum or ferric sulfate; and/or 2) an electrolytically active polymer.
Polymers are long-chain, high-molecular-weight, organic polyelectrolytes. There are several reasons for considering the use of polymers: 1) increased settling rate and high shear strength; 2) production of a smaller volume of sludge; and 3) easier dewatering. Also, polymers have a minor effect on pH; consequently, the need for final pH adjustment in the finished water may be reduced.
There are three polymer classifications: 1) cationic; 2) anionic; and 3) nonionic. Cationic polymers, when dissolved, produce positively charged ions. They are widely used because the suspended and colloidal solids commonly found in water are negatively charged. Cationic polymers can be used as the primary coagulant or as an aid to coagulants such as alum or ferric sulfate. Anionic polymers are polymers that dissolve to form negatively charged ions, and are used to remove positively charged solids. The anionic polymers increase floc size, improve settling, and generally produce a stronger floc. They are not materially affected by pH, alkalinity, hardness, or turbidity.
Nonionic polymers are polymers having a balanced or neutral charge, which, upon dissolving, release both positively and negatively charged ions. Nonionic polymers may be used as coagulants or as coagulant aids.
Coagulation
When a coagulant is added to water, destabilization of the colloids occurs and a microfloc forms. The interactions are: 1) particle coalescence; 2) the aggregation of particles by interparticulate bridging between reactive groups on the colloids; and 3) the enmeshment of particles in the precipitate floc that is formed. The agitation of the water is also important in this type of aggregation, since it causes interparticulate contacts.
Flocculation/Coagulation
Flocculation is the use of organic polymers to provide further agglomeration of particles to produce large, stable, easily settled particles.
Mixing
Mixing is an important unit operation in flocculation/coagulation. For this application simplicity and reliability of operation are critical and lead to consideration of static mixers, particularly in-line static mixers, which contain elements that bring about sudden changes in the velocity patterns as well as momentum reversals. In-line static mixers are commonly used for the mixing of chemicals.
Operation of the Coagulation/Flocculation Process
The selection of chemical coagulant and coagulant aids is a continuing program of trial and evaluation, normally using the jar test. To do a thorough job of chemical selection, the temperature, pH, alkalinity and turbidity of the water to be treated should be considered.
The initial selection of chemicals to be tested derived from the water treatment background of one of the investigators, who has over ten years experience in water and wastewater treatment both conventional and nonconventional and was based on the knowledge that charge neutralization must first take place to allow nucleation of the colloids (initiation of zone settling). The next step would then be a polymer of high molecular weight to collect the nucleated colloids and initiate compression settling.
The effectiveness of a coagulant will change as raw water characteristics change. The effectiveness of coagulant and coagulant-aid chemicals may also change for no apparent reason, suggesting that there are other factors, not yet understood, that affect coagulation and flocculation. Because of differences in the characteristics of the suspended matter found in natural waters, not all waters can be treated with equal success with the same polymer(s) or the same dosages. In selecting the best materials for each water supply, jar tests are run with several dosages of various polymers, with due consideration of cost versus performance.
The jar test, although still the most widely used coagulation control test, depends on the human eye for evaluation and interpretation. To gain further information, pH, turbidity tests, zeta potential and perhaps filterability tests are also useful. Of these tests, we performed the pH and turbidity tests. Of these tests, we performed the pH and turbidity tests.
Jar test results help determine the type of chemicals to use and dosage ranges. Since restabilization due to overdosing can easily occur when organic polymers are used, it is necessary when performing jar tests to vary the polymer dosage over a range of concentrations.
Although the jar tests provide a good indication of the results to expect, full-scale plant operation may not always match these results. For this reason additional testing may be done with a bench scale system to further refine design criteria and expected full-scale performance.
EXPERIMENTAL APPARATUS
Two separate data collection activities were performed in this study: 1) Jar test experimental parameters defined the coagulants/polymers used for the bench scale experiments; and 2) bench scale experimental parameters were developed to help define the guidelines for applying coagulants/polymers to the basin.
Jar Test Experiment
Jar test experiments provided data that assisted in determining the appropriate coagulant and/or polymer to use with the basin water. These experiments were performed using basin water taken from five sections of the basin. The parameters of interest are:
An experiment was initiated by measuring the pH and turbidity of basin water before starting the jar tests. A 1000-ml sample of basin water was then placed in a beaker and placed on a jar stirrer. Up to six jar tests could be performed simultaneously. A coagulant and/or polymer dosage, at a selected concentration was added to the beaker while simultaneously mixing the contents at a fixed speed. After a set time, the stirrers were shut off and the floc formed was allowed to settle. Turbidity values were then obtained every five minutes, and a final pH was measured at the end of the experiment. Initially, turbidity was measured for 30 minutes, but were changed to 15 minutes due to the lack of change during the extended measurements. During the settling period, the flocculent was observed and manipulated to determine settling and strength characteristics.
The jar tests were performed using a Phillips & BirdTM Jar Tester equipped with 6 paddle stirrers, an illuminated base, and variable speed control. The jar tester has the capability of performing up to 6 tests at a time, with speeds ranging from 0-320 revolutions per minute. The jar tester is approximately 3 feet long, 0.5 feet wide, and 1.5 feet high. The pH was measured using a HACHTM EC10 Portable pH/mV/Temperature meter, with automatic slope calibration.
Turbidity values were measured using a microprocessor-controlled HACHTM 21 00P Turbidimeter. The 21 00P provides direct digital readout in nephelometric turbidity units (NTUs) with three manual range modes: 0 to 9.99, 0 to 99.9, or 0 to 1000 NTU. Calibration is based on formazin, the accepted primary standard for turbidity measurements, with routine verification of accuracy, a set of Gelex secondary standards were used.
Bench-Scale Experiment
Bench-scale tests provided data that assisted in determining four things: I) how the coagulants and/or polymers would react under the stress of a high shear pump; 2) the effectiveness of in-line mixers for mixing chemicals; 3) the optimum polymer dosages to; and 4) the preferred injection point of chemicals. These experiments were performed using basin water taken from five sections of the basin
A successful bench scale experiment would yield:
The turbidity of the basin water samples was measured before starting the bench-scale test. The 5-gallon samples from various parts of the NBasin were in carboys. For each test a carboy placed upstream of the bench-scale unit. Multiple tests were performed with each 5-gallon sample. A high shear pump processed the basin water sample. A fixed dosage and concentration of polymer was injected with a metering pump and mixed with the basin water thru in-line mixers. The in-line mixers were placed in two configurations around the high shear pump to determine the effects of the pump on the chemicals. The fast configuration consisted of one in-line mixer upstream and one downstream of the high shear pump. The chemicals were pumped thru both the upstream and downstream in-line mixers. The second configuration consisted of both in-line mixers downstream of the pump. After allowing the system to stabilize a sample was taken from the discharge and observed for settling rate and other visual characteristics. A turbidity reading was then made. Experiments were run for 10-15 minutes.
The bench-scale experiments were performed with the pump and metering system shown in Fig. 2. The bench-scale apparatus is equipped with two metering pumps, two in-line mixers, one high shear pump, four open/close valves, two check valves, and four containers for the basin water, chemicals, and treated material. The majority of fittings and connections are Swageloc. The bench-scale apparatus is approximately 2 feet wide by 3 feet long, and is mounted on a plywood board.
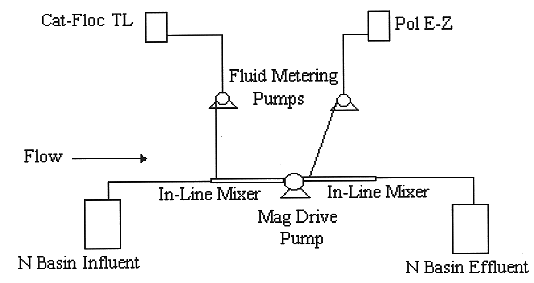
Fig. 2. Bench scale
test apparatus to meter polymer with in-line mixers.
The basin water sample was processed thru the in-line mixers using a Micropump high-shear pump. The Micropump pump head features an enclosed gear assembly, and drive magnets that are encapsulated in 316 stainless steel and Teflon for durability and chemical resistance. Flows range is from 0-2460 ml/min. An internal bypass valve recirculates excess discharge pressure to the inlet side of the pump to protect the pump head and entire system from excessive pressures.
The chemicals were injected thru the in-line mixers using a Fluid Metering Inc.TM single head, fixed-speed AC drive metering pump. The metering pump features interchangeable drives with a variety of pump heads. A flow adjustment is available to set flow from 0 to 100% of maximum flow rate at greater than ± 1% accuracy.
The basin water and chemicals were mixed using two Koflo Stratos TubeTM in-line mixers. Both the mixing elements and housing are constructed of 316 stainless steel. All elements are fixed throughout the housing by a nickel base alloy. Tube mixers are supplied with plain ends and accept standard tube fittings.
The turbidity was measured using the same HACH instrument described in the Jar Test Experiment section of this report.
RESULTS
Jar Test Experiment
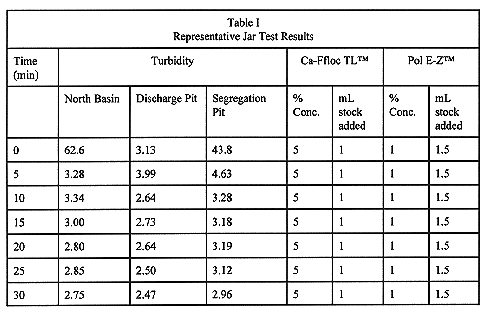
Approximately 60 jar tests were performed. The best results (Table II) were with a 5% solution of Cat-Floe TLTM and a 1% solution of Pol E-ZTM (Calgon Corp.). The Cat-Floc TLTM is a cationic polymer that performs the same charge neutralization function as a coagulant. The Pol E-ZTM is a anionic polymer that performs the function of a coagulant aid. The results were consistent with samples from different sections of the basin as summarized in Table I. Changes in pH were minimal ranging from 0.05 to 0.24.
Table I Representative Jar Test Results
Bench Scale Experiment
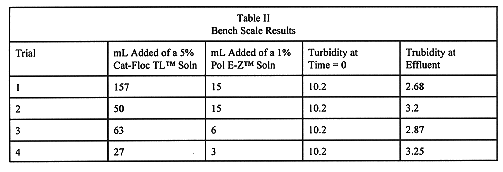
Approximately 45 bench scale tests were performed. Of the two configurations possible, the one with an in-line mixer both before and after the pump was used for the results reported. Brief tests with both in-line mixers, and both polymers injected, downstream of the pump did not show improved results thus indicating that the pump was not physically degrading the Cat-Floe TLTM. The best results in terms of most reduction in NTUs with the least amount of polymers were at a dosage of 27 parts-per-million (ppm) of 5% Cat-Floe TLTM, and 3 ppm of 1% Pol E-ZTM (CalgonCorp.). These results were obtained with one in-line mixer upstream (vacuum side), and one downstream (pressure side) of the high shear pump (Table II). Visually, the solids settled within several minutes. Repeat measurements of NTUs at five minute intervals for 30 minutes showed no further significant changes.
Table II Bench Scale Results
DISCUSSION
Jar Test Experiment
As discussed in the theory section of this report, the selection of chemical coagulant and coagulant aids is a continuing program of trial and evaluation, normally using the jar test. Approximately 60 jar tests were used to select the chemicals and provide starting dosage rates for the bench scale test, which, in turn, will support application to the NBasin.
Experimental parameters defined the chemicals used for the bench scale tests. The jar test experimental parameters were determined by a combination of reviewing theory and satisfying customer requests. The results of these experimental parameters provided information that resolved the jar test goals. The goals of the jar test and the results are presented in Table III.
Table III Jar Tests Results Summary
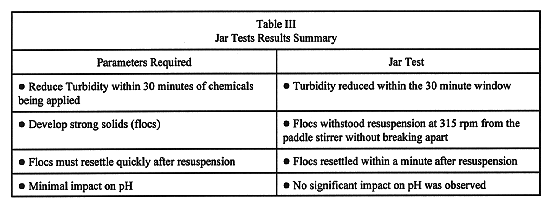
Reducing the turbidity within 30 minutes was the most important parameter for finding a coagulant and polymer. With various basin cleanup activities in progress and an aggressive work schedule to complete, reducing turbidity quickly is important. Cleanup activities tend to resuspend settled solids. During resuspension, basin visibility and work production drops significantly. Within a few days solids will resettle. However, since this settling rate is unacceptable for obtaining scheduled milestones, the chemicals selected have to re-establish visibility by quickly resettling solids and reducing turbidity.
Turbidity reduction is shown in Fig. 3. The beaker on the left illustrates high turbidity basin water (over 60 NTU), while the beaker on the right is treated basin water (under 3.0 NTU). This reduction occurred in approximately 5 minutes.

Fig. 3. Turbidity reduction in jar
tests.
When the flocculent (solids) created by the coagulant and polymers is resuspended, it must withstand high shear forces and resettle quickly. If the flocculent breaks up and doesn't resettle, additional chemicals will have to be added to re-establish the flocculent. Therefore, understanding floc strength and resettling properties was important for selecting a coagulant and polymer.
For basin activities, such as protecting equipment and controlling biological growth, maintaining pH is important. It was then, important to find a polymer that would be compatible with and maintain a basin pH between 6.5 and 7.5, while providing efficient coagulation/flocculation Therefore, a goal with the jar tests was to find a coagulant and/or polymer that would be active and maintain the required pH range.
The mixing time was also important in selecting a coagulant and coagulant aid. The initial seconds of mixing are considered the most crucial in floc development. The quicker the mixing time maximizing random collisions, the better the floc development. Also, the longer it takes the floes to develop, the longer it could take to improve visibility. This, in turn, impacts schedules. The ideal chemicals should react and form floes quickly.
Another important factor in selecting a coagulant or flocculent is to determine the consistency of the response. This was done by performing jar tests with samples from five different sections of the basin.
Based on the criteria discussed above, the coagulant and flocculent (both polymers) selected for the bench scale tests were Calgon products Cat-Floe TL and Pol E-Z 7736. This combination reduced turbidity to under 3.0 NTU within the goal of 30 minutes of injection. Once the floes were resuspended, they resettled quickly and were resistant to breaking. The flocs formed quickly after the chemicals were mixed with the sample. The flocs were solid brownish clumps, with no signs of cloudiness. The water was very clear. The above jar test results were consistent with samples taken from the Segregation Pit, Discharge Pit, View Pit, North and South Basins of the N Reactor Fuel Storage Basin.
Bench Scale Experiment
Approximately 45 bench scale tests were used to determine four parameters: 1) how the Cat-Floc TL would react under the stress of a high shear pump; 2) the effectiveness of in-line mixers for mixing chemicals; 3) the optimum dosages of Cat-Floe TLTM and Pol E-ZTM to use; and 4) the preferred injection point of chemicals.
The rapid mixing of chemicals in a liquid can be carried out in a number of different ways, including static in-line mixers. For the basin application, it is anticipated that in-line mixers will be required to provide the appropriate amount of mixing and the simplest equipment. Therefore, bench scale effectiveness of in-line mixers for mixing chemicals was important to substantiate.
It is also important to minimize the amount of organic material used in the basin since there are limits on organic carbon in sludge sent for disposal. The bench scale tests were used to define the minimum amounts of Cat-Floe TLTM and Pol E-ZTM required for effective basin treatment.
Polymers such as Cat-Floc TLTM, are short chain organic compounds which are less susceptible to breaking under the stress of rapid mixing. Polymers such as POL-E-ZTM, are long chain, high molecular weight organic compounds that are easily susceptible to disintegration under the stress of mixing. How much stress a chemical receives is dependent on where it is injected relative to a high shear regimes. Injecting the Cat-Floe TLTM upstream of the pump would be preferred for mixing effectiveness but must be evaluated against the risk of physical degradation. Rapid mixing ranges from a fraction of a second to about 30 seconds. Improper mixing can often be corrected by altering the point of injection. The injection points were evaluated with the bench scale tests by changing the configuration of the in-line mixers around the high shear pump as previously discussed. Limited results demonstrated that the Cat-Floe TLTM survived the high shear of the gear pump and that was maintained as the preferred configuration.
As with the jar test, visually observing the sample was important in determining the effects of the high shear pump and in-line mixers on the floc development.
The pH was not evaluated because it was believed that it would not be different from the jar test results. The bench scale test was performed with samples from only one location in the basin because results were expected to be similar with other samples.
CONCLUSIONS AND RECOMMENDATIONS
Based on the criteria discussed above, a 27 part-per-million (ppm) solution of 5% Cat-Floe TLTM and a 3 ppm solution of 1% Pol E-ZTM was determined the most efficient combination. The turbidity was reduced to slightly above 3.0 NTU within 10 minutes. The reason the higher turbidity reading was accepted is because the results were obtained with a minimal addition of chemicals. The flocculent selected and the configuration of the high shear pump and in-line mixers provided injection points that allowed the Cat-Floe TLTM and Pol E-ZTM to produce properly formed flocculent. Once the flocculent was resuspended, it resettled quickly and was resistant to breaking. The flocculent formed quickly after chemicals were mixed with the sample.
To implement this treatment in the Nbasin, a pumping and piping skid should be constructed that consists of two in-line mixers and a pump. The Cat-Floe TLTM should be injected upstream of the pump and pass through an in-line mixer downstream of the pump. The Pol E-ZTM should then be injected and pass through the second in-line mixer. In terms of mixing this is equivalent to the configuration of in-line mixers and the pump used in the bench test for the results reported. This system should be designed for submerged operation to provide shielding. A chemical feed system should be provided to inject the polymers into appropriate locations on the skid. This feed system can be above water for convenient operation since the materials handled are non-radioactive. Sampling ability should be provided to measure treated water turbidity.
Due to the large scale up factor, over 1000, the entire system may require additional testing and development to confirm performance and fine tune polymer injection rates before deployment to treat the bulk of the Nbasin water.
REFERENCES