
Fig. 1. Diagram of the WIPP repository in situ, showing a drilling scenario.
M. S. Y. Chu, H. W. Papenguth, C. T. Stockman, Y. Wang, R.
F. Weiner
Sandia National Laboratories
G. Basabilvazo
U. S. Department of Energy
ABSTRACT
This paper presents some features of DOE's demonstration of compliance with the EPA regulation of the Waste Isolation Pilot Plant (WIPP). Performance of the WIPP as a repository requires that releases to the accessible environment not exceed the limits of the regulation 40 CFR Part 191(1) either when the WIPP is undisturbed, or if there is intrusion into the repository by drilling. This paper addresses only the cumulative radionuclide release limits, and does not address individual or groundwater dose limits of 40 CFR Part 191. The radioisotopes that may influence repository performance are Pu-239, Pu-240, Pu-242, Am-241, U-233, U-234, Th-229, and Th-230. Other waste characteristics and waste components that have a significant impact on repository performance are waste shear strength; the solubility of plutonium and americium compounds in the waste; iron that reduces the actinides to less soluble oxidation states and corrodes to produce hydrogen gas; cellulose, plastic, rubber, nitrate, and sulfate, that microbes can metabolize to methane to increase gas pressure and that can form colloidal particles; humic materials that form colloidal particles; nonferrous metals that prevent increases in actinide solubility by binding with organic ligands in the waste.
INTRODUCTION
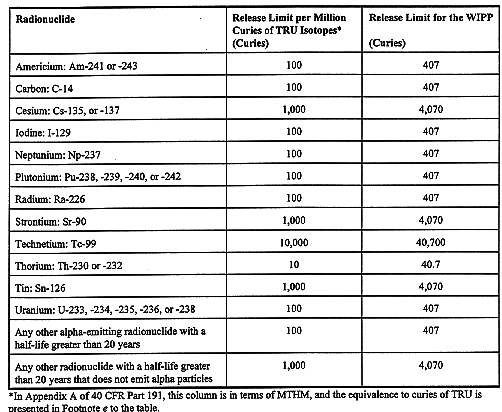
This paper presents some features of DOE's demonstration of compliance with the EPA regulation of the Waste Isolation Pilot Plant (WIPP). The WIPP, a geologic repository for transuranic (TRU) waste, is located 2150 feet below the ground surface in a bedded salt formation about 20 miles east of Carlsbad, NM. A diagram of the WIPP is shown in Fig. 1. Performance of the WIPP as a repository requires that releases to the accessible environment not exceed the limits of the regulation 40 CFR Part 191(1) either when the WIPP is undisturbed, or if there is intrusion into the repository by drilling. This paper addresses only the cumulative radionuclide release limits, and does not address individual or groundwater dose limits of 40 CFR Part 191. Table I summarizes the regulatory release limits.

Fig. 1. Diagram of the WIPP repository in situ, showing a drilling scenario.
Table I Table 1 of Appendix A, 40 CFR Part 191
In 1996, the EPA promulgated 40 CFR Part 194(2): the implementing regulation for 40 CFR Part 191. The regulatory subsection addressed here, 40 CFR 194.24(b), directs the DOE to identify and analyze the components and characteristics of the TRU waste that can impact performance of the WIPP repository, and thereby possibly impact waste containment. DOE must also analyze those waste characteristics and components that will not affect repository performance.
"Affecting repository performance" means affecting the ability of the repository to isolate TRU waste for 10,000 years. The characteristics of the waste that can affect performance are:
THE IMPACT OF RADIOACTIVITY ON REPOSITORY PERFORMANCE
EPA regulates repository performance by allowing release of a fraction of the radionuclide inventory, as is illustrated by Table I. Therefore, this inventory impacts performance in two ways. The first is in determination of the "waste unit factor" -- the normalization factor, or denominator of the fraction. The waste unit factor is the inventory, at closure, of transuranic isotopes that are alpha emitters and have half lives longer than 20 years, and has been analyzed by DOE to be 3.44 (corresponding to an inventory at closure of 3.44 million curies). The radionuclides included in the waste unit factor are the long-lived isotopes of plutonium, neptunium, americium, and curium in the inventory. 99.9% of the waste unit factor consists of the inventory of Pu-238, Pu-239, Pu-240, and Am-241. The WIPP differs markedly from a high-level radioactive waste (HLW) repository because the radionuclides in the waste unit factor (the fraction denominator) are, for the most part, the same as those used in calculating potential releases (the numerator), rendering accuracy in determining the waste unit factor less critical to performance assessment calculations. For a HLW repository, the fraction numerator is much more heavily influenced by fission products, not TRU isotopes, and the waste unit factor is relatively more important.
The second impact is on modeling or calculation of possible releases, and potentially includes all radionuclides in the repository. However, most of these are present in the waste in such small quantities that their impact on long-term performance is negligible For a mix of radionuclides, as in the WIPP, each radionuclide is normalized with respect to its release limit (1), and the sum of all releases must have
where the sum of releases in EPA units expressed by
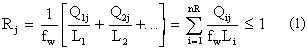
in which Rj is the total release in EPA units under scenario j, fw is the waste unit factor, Qij is the cumulative release for radionuclide I under scenario j, Li is the EPA release limit for radionuclide I, and the EPA release limit nR is the number of radionuclides contributing to the release. The EPA unit provides a more precise description of waste radioactivity than a unit like curies or becquerels, because it addresses regulatory compliance directly. The calculation of possible releases and the associated probabilities of release (under various scenarios) is presented as a complementary cumulative distribution function (CCDF), as is shown in Fig. 2.

Fig. 2. Mean CCDFs for specific release modes.
A number of different intrusion scenarios were considered in assessing WIPP performance. Of these, the most severe intrusion is by drilling into the repository. Drilling essentially dominates other intrusion scenarios, and is therefore the only one included in the WIPP Compliance Certification Application (CCA). Radionuclides can be released by drilling in three different ways: (1) by direct release in the drill mud, (2) by direct brine release on drilling, and (3) by release of brine to an overlying stratum. Different radionuclides are involved in each of these release modes.
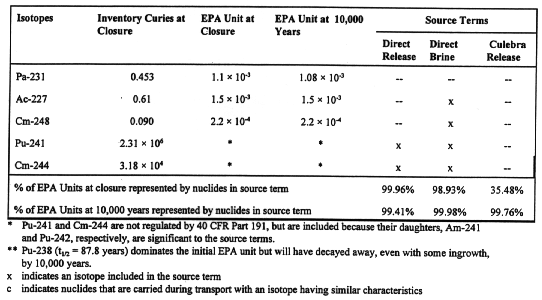
Modeling of direct release by drilling (including caving) includes Pu-238, Pu-239, Pu-240, Am-241, Cs-137, Sr-90, U-233, and U-234. The isotopes Pu-241 and Cm-244, although not regulated by 40 CFR Part 191, are included because their daughters, Am-241 and Pu-240, respectively, are significant. Direct release of brine to the surface can result in the release of isotopes mobilized in the brine. In modeling this type of release, the isotopes of thorium, uranium, neptunium, plutonium, americium, and curium present in the waste are included.
All isotopes of an a particular actinide exhibit the same solubility, and can therefore be assumed to dissolve in the same mole proportion as the inventory, which is essentially the same mass proportion because the masses are similar. That is, if the uranium in the repository is 85% U-238 by weight, 85% of any dissolved uranium will also be U-238. U-238 has an exceedingly long half life (4.9 billion years) and its specific radioactivity is very low (3 x 10-7 Ci/gm) compared to, for example, U-233 (about 300,000 Ci/gm). In a solution, a preponderance of U-238 will effectively dilute the radioactivity of other isotopes in that solution.
Brine released to an aquifer in an overlying stratum like the Culebra aquifer (see Fig. 1) can also carry mobilized radionuclides. The eight radionuclides used in calculation of brine release that dominate the total potentially released EPA unit for all but the earliest part of the regulatory period are Pu-239, Pu-240, Pu-241, Pu-242, Am-241, U-233, U-234, Th-229, and Th-230.
In sum, the radioisotopes that may influence repository performance are Pu-239, Pu-240, Pu-242, Am-241, U-233, U-234, Th-229, and Th-230; for potential releases in the first few hundred years of the regulatory period, Pu-238 , Sr-90, and Cs-137 are included in the calculation (Table II). When potential releases are modeled, however, the only radioisotopes that appear to be significant are Pu-239 and Pu-240 throughout the regulatory period, and Pu-238 and Am-241, during the first thousand years after closure.
Table II Isotopes Included in Source Terms

Table II (contd.) Isotopes Included in Source Terms
IMPACT OF OTHER WASTE CHARACTERISTICS AND COMPONENTS ON REPOSITORY PERFORMANCE
Characteristics (and components) of the waste that can enhance radionuclide mobility can thereby influence repository performance. Figure 2 shows CCDFs for direct release of brine to the surface, as well as direct releases to the accessible environment if the WIPP were inadvertently penetrated by drilling. According to EPA's definition (2), the accessible environment is not only the ground surface, but any location outside the four-square-mile lateral cross-section centered on the repository (2) (see Fig. 1). Release to the accessible environment therefore includes release to an aquifer in a formation that overlays the WIPP--the Culebra aquifer, in this case (Fig. 1). As the legend of Fig. 2 indicates, the mean CCDF for subsurface releases (to the Culebra aquifer) does not appear because those potential releases are so low that they are off the scale of the figure.
The amount of radionuclides released in brine depends on both the brine volume and the concentration of radionuclides dissolved or otherwise mobilized in the brine. The amount of radionuclides released on direct drilling depends on waste strength, waste particle size, and gas pressure in the repository. Since relatively small brine volumes are postulated for direct release, the characteristics that have the most potential effect on repository performance are those that affect direct release of radionuclides by drilling.
Characteristics That Influence Direct Drilling Release
The amount of material released directly on drilling depends on the erosion of the waste at the time of the intrusion. This erosion depends in turn on the shear strength and compressibility of the waste. Waste components that contribute to gas pressure can also affect this type of potential release; if the breached repository is under pressure, material can be forced out by such pressure.. Anoxic corrosion produces hydrogen gas and microbial action produces methane, both of which contribute to increased gas pressure, which may reach lithostatic (3).
Characteristics That Influence Radionuclide Mobilization
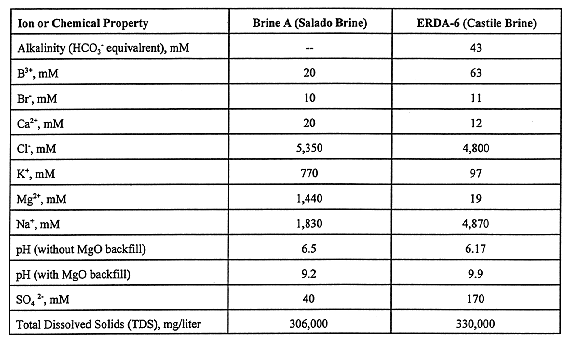
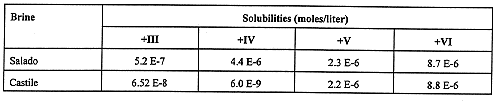
Mobility of radionuclides transported in brine is a function of both solubility and the tendency to form or sorb to colloidal particles. Under possible intrusion scenarios, brines of two different compositions can inundate the repository: the Salado brine, which contains considerable MgCl2, and brine from a reservoir atop the Castile Formation (see Fig. 1): a NaCl brine. Brine compositions are given in Table III (4). Cellulosic materials, plastic, rubber, iron and steel, organic ligands, and, to a small extent, nitrate, can affect actinide solubility in these brines. It is estimated that all cellulosic material in the waste (and about half of the plastics and rubber) can be metabolized to CO2 , methane, and other gases by the action of halophilic bacteria. CO2 is the only one of these gases that can significantly affect actinide solubility. The existence of viable halophilic bacteria in the WIPP after closure is uncertain, so that for modeling purposes such bacteria are assumed to be present half of the time. CO2 is produced only if bacteria are present. The hydrogen produced by anoxic corrosion in the absence of bacteria has no detectable impact on solubility.
Table III Some Chemical Components of WIPP Brines.
In an unbuffered system, dissolved CO2 would decrease the pH and increase solubility, depending on other constituents in the solution. However, MgO backfill will react with and sequester any dissolved CO2 as anhydrous or hydrated MgCO3, and will be present in the WIPP repository in sufficient excess that its reactions will buffer the pH at 9.2 in Salado brine and 10 in Castile brine (Fig. 3). In such alkaline brine, actinides are relatively less soluble (5) although solubilities can vary from about 10-11 M to about 10-4 M (6). The most likely range of actinide solubilities is between about 10-8 M and about 10-5 M (Table IV).
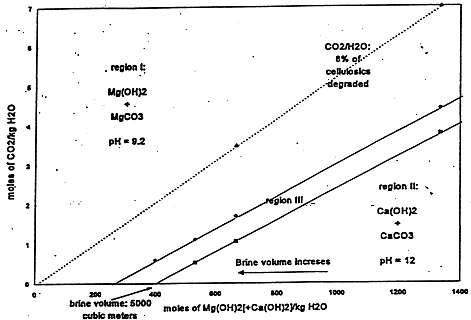
Fig. 3a. Chemical Buffer distribution for Salado brine.
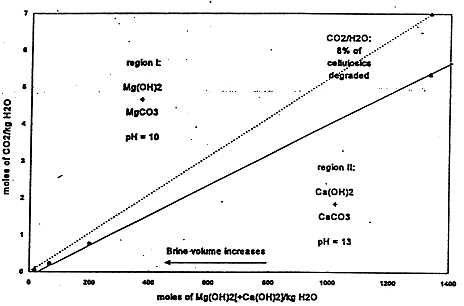
Fig. 3(b). Chemical buffer distribution for Castile brine.
Table IV Modeled Solubilities of the Actinide Oxidation States, in Moles/Liter, With MgO Backfill
In addition to pH and CO2, solubility is most directly affected by redox conditions, that are controlled largely by iron and steel waste containers and component. Iron and ferrous ion can reduce actinides to lower, less soluble oxidation states chemically, under anoxic conditions. The spectra of Fig. 4 show the reduction of Pu(+6) to Pu(+4) by iron.

Fig. 4. Reduction of Pu(VI) by iron in 0.1 M NaCl and in Castile (ERDA-6) brine.
Dissolution of the actinides could be enhanced directly by the formation of soluble complexes, including chelates. The waste contains four water-soluble organic ligands: acetate, citrate, oxalate, and ethylene diamine tetra-acetate (EDTA); these become part of the waste because they are used as cleaners and degreasers in plutonium processing. However, other metal species that are available to bind organic ligands include iron, nickel, chromium, vanadium, and manganese, because the steels used for the waste drums contain these as constituents.
A temperature increase could affect performance by increasing actinide solubility, and could result from both expected exothermic chemical reactions (corrosion and hydration of MgO) and radioactive decay. A temperature increase from both of these sources is not expected to exceed 5° C (7). Although pressure in the repository is expected to reach lithostatic pressure if there is no intrusion, and drop to hydrostatic pressure in the event of human intrusion, pressure does not influence solubility until it is at least an order of magnitude higher than lithostatic pressure.
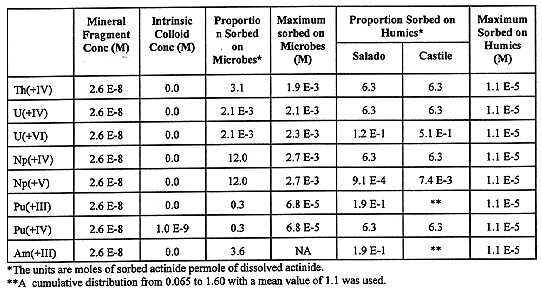
Actinides can also be mobilized in colloidal form as intrinsic colloids, or sorbed on nonradioactive colloidal particles in the waste. Plutonium in the waste can form an intrinsic colloid, and the microbial breakdown products of cellulosic materials, plastic, and rubber can contribute to the quantity of humic colloids. Within the repository, mineral fragment colloids could form from corrosion of iron-bearing waste, the steel packaging materials, and portland cement-based matrices, but are expected to be kinetically destabilized in the high-ionic strength brines present in the disposal room, and their contribution to actinide mobility is negligible. Humic colloids will be present in the repository, both in soil and as cellulose breakdown products. Cellulose, plastic, and rubber in the waste, as well as nitrate, are microbe nutrients, and consequently increase the microbe population. Microbial and humic colloids can transport concentrations of actinides that are several multiples of the dissolved concentration, and thus increase the potential for actinide mobility considerably. Table V gives these proportions.
Table V Colloid Concentration Factors
The following calculation of total mobilized actinide illustrates the impact of solubility and colloid concentration on repository performance.
(2) Total Mobile Actinide Concentration = Dissolved + Humic Colloid + Microbial Colloid + Mineral Fragment Colloid + Intrinsic Colloid
The dissolved concentration is calculated by the FMT code (6). The other terms are taken from Table IV:
(3) Humic Colloid = Dissolved * Proportionality Constant if Dissolved *Prop. Const. < Humic Cap, otherwise Humic = Humic Cap
(4) Microbe = Dissolved * Proportionality constant if the Total Mobile < Microbe Cap, otherwise Microbe = Microbe Cap
Mineral and Intrinsic Colloid directly from Table V
For example, for one realization, in Salado brine, Pu would be present in the +IV state, exhibiting a model solubility of 4.4 x 10-6 mole/liter. The humic proportionality constant for the +IV oxidation state in Salado brine is 6.3, the microbe proportionality constant for Pu is 0.3, the humic cap is 1.1x10-5 M, the microbe cap for Pu is 2.1x10-3, the actinide on mineral fragment concentration is 2.6 x 10-8, and the Pu intrinsic colloid concentration is 1x10-9.
The humic complexed plutonium would be:
(5) (4.4 x 10-6 mole/liter)(6.3 moles bioaccumulated per mole) = 2.8 x 10-5 moles/liter
This value, however, exceeds the cap for humic-mobilized plutonium, 1.1 x 10-5 mole/liter. Therefore, in this case, the cap would be used for the humic mobilized actinide concentration.
The microbial mobilized plutonium would be:
(6) (4.4 x 10-6 mole/liter)(0.3 moles adsorbed per mole/liter) = 1.3 x 10-6 moles
which is less than the cap.
The total plutonium concentration for this realization would then be the sum of the dissolved and colloidal actinides:
(7) 4.4 x 10-6 + 1.1 x 10-5 + 1.3 x 10-6 + 2.6 x 10-8 + 1.0 x 10-9 = 1.7 x 10-5 mole/liter
Actinide solubility affects performance, and humic colloids also may be significant. Microbial colloids can have an effect, but are not waste characteristics. Intrinsic plutonium colloids and mineral fragment colloids have relatively very little impact on performance. Because the MgO backfill essentially controls the chemistry of the brine-inundated repository, the solubility is primarily a function of the brine type (Salado or Castile) and the iron compounds (largely the waste containers) that control the redox environment. The waste constituents with potentially significant impact on repository performance are therefore iron compounds, humic substances like soil, and the humic detritus from microbial metabolism.
SUMMARY AND CONCLUSIONS
The waste characteristics and waste components that have a significant impact on repository performance are:
Iron and iron compounds, and non-ferrous metals have clearly beneficial effects by reacting to reduce actinide mobility. Very low activity isotopes have a beneficial effect by diluting activity in solution. Increased gas pressure can have a beneficial effect by keeping brine out of the repository, or a detrimental effect on direct drilling releases. Humic materials that sorb actinides colloidally can have a detrimental effect by their enhanced mobility in brine. The effect of waste characteristics on repository performance can thus be either beneficial or detrimental.
REFERENCES